Testsealabs MTD Methadone Test Drug of Abuse DOA Test
[INTRODUCTION]
Methadone is a narcotic pain reliever for medium to severe pain. It is also used in the treatment of heroin (opiate dependence: Vicodin, Percocet, morphine, etc.) addiction. Oral methadone is very different than IV methadone. Oral methadone is partially stored in the liver for later use. IV methadone acts more like heroin. In most states you must go to a pain clinic or a methadone maintenance clinic to be prescribed methadone.
Methadone is a long acting pain reliever producing effects that last from twelve to forty-eight hours. Ideally, methadone frees the client from the pressures of obtaining illegal heroin, from the dangers of injection, and from the emotional roller coaster that most opiates produce. Methadone, if taken for long periods and at large doses, can lead to a very long withdrawal period. The withdrawals from methadone are more prolonged and troublesome than those provoked by heroin cessation, yet the substitution and phased removal of methadone is an acceptable method of detoxification for patients and therapists.
The MTD Methadone Test (Urine) yields a positive result when the concentration of methadone in urine exceeds 300ng/ml.
[Materials Provided ]
1.FYL Test Device (strip/cassette/dipcard format)
2. Instructions for use
[Materials required, not Provided]
1. Urine collection container
2. Timer or clock
[Storage Conditions And Shelf Life]
1.Store as packaged in the sealed pouch at room temperature (2-30℃ or 36-86℉). The kit is stable within the expiration date printed on the labeling.
2.Once open the pouch, the test should be used within one hour. Prolonged exposure to hot and humid environment will cause product deterioration.
[Testing Method]
Allow the test and urine samples to equilibrate to room temperature (15-30℃or 59-86℉) prior to testing.
1. Remove the test cassette from the sealed pouch.
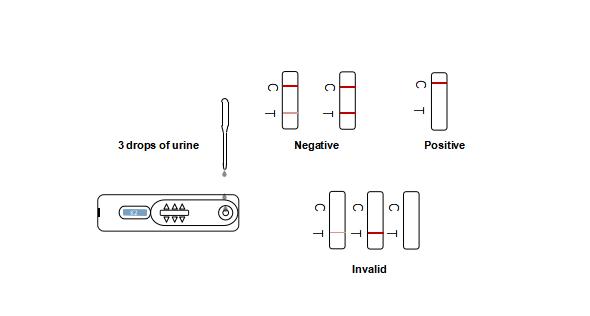
2. Hold the dropper vertically and transfer 3 full drops (approx. 100ml) of urine to the specimen well of the test cassette, and then begin timing. See the illustration below.
Wait for colored lines to appear. Interpret the test results at 3-5 minutes. Do not read results after 10 minutes.
[Materials Provided ]
1.FYL Test Device (strip/cassette/dipcard format)
2. Instructions for use
[Materials required, not Provided]
1. Urine collection container
2. Timer or clock
[Storage Conditions And Shelf Life]
1.Store as packaged in the sealed pouch at room temperature (2-30℃ or 36-86℉). The kit is stable within the expiration date printed on the labeling.
2.Once open the pouch, the test should be used within one hour. Prolonged exposure to hot and humid environment will cause product deterioration.
[Testing Method]
Allow the test and urine samples to equilibrate to room temperature (15-30℃or 59-86℉) prior to testing.
1. Remove the test cassette from the sealed pouch.
2. Hold the dropper vertically and transfer 3 full drops (approx. 100ml) of urine to the specimen well of the test cassette, and then begin timing. See the illustration below.
3. Wait for colored lines to appear. Interpret the test results at 3-5 minutes. Do not read results after 10 minutes.
[Results interpretation]
Negative: *Two lines appear. One red line should be in the control region (C), and another apparent red or pink line adjacent should be in the test region (T). This negative result indicates that the drug concentration is below the detectable level.
*NOTE: The shade of red in the test line region (T) will vary, but it should be considered negative whenever there is even a faint pink line.
Positive: One red line appears in the control region (C). No line appears in the test region (T). This positive result indicates that the drug concentration is above the detectable level.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test panel. If the problem persists, discontinue using the lot immediately and contact your local distributor.
[You may be interesting in the products information belows]
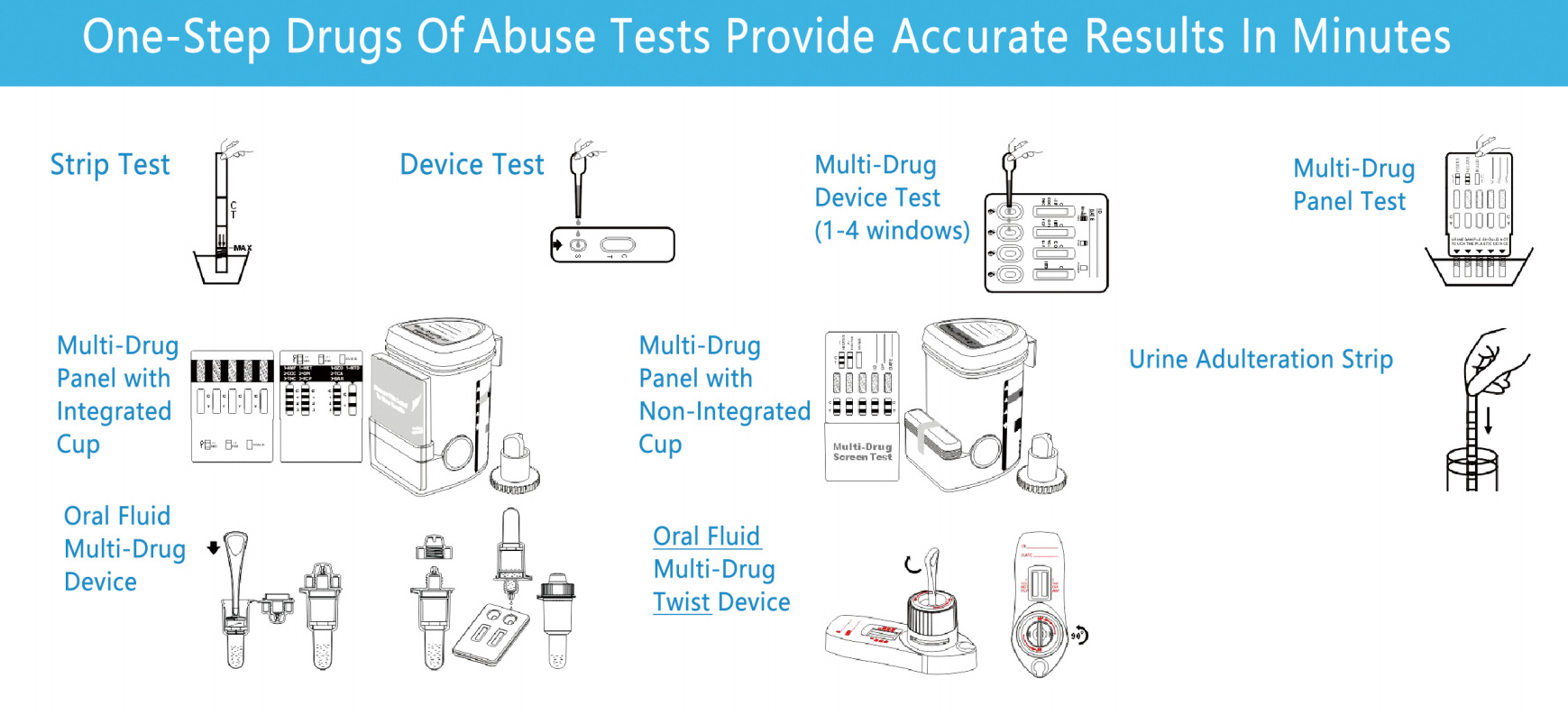
TESTSEALABS Rapid Single/Multi-drug Test Dipcard/Cup is a rapid, screening test for the qualitative detection of single/multiple drugs and drug metabolites in human urine at specified cut off levels.
* Specification Types Available
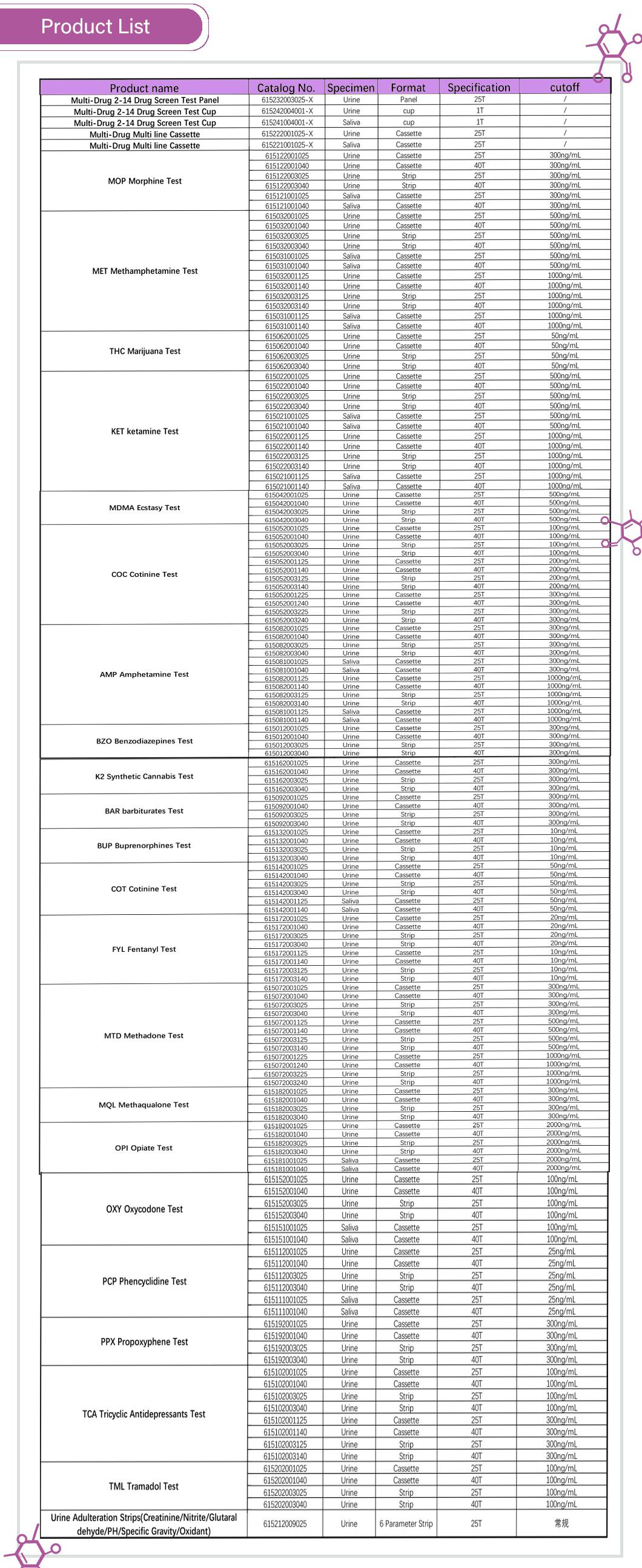
√Complete 15-drug product line
√Cut-off levels meet SAMSHA standards when applicable
√Results in minutes
√Multi options formats--strip,l cassette , panel and cup
√ Multi-drug device format
√6 drug combo( AMP,COC, MET, OPI, PCP, THC)
√ Many Different combinations available
√ Provide immediate evidence of potential adulteration
√6 Testing parameters: creatinine, nitrite, glutaraldehyde, PH, Specific gravity and oxidants/pyridinium chlorochromate