Testsea Disease Test HBsAg Rapid Test Kit
Quick Details
|
Brand Name: |
Testsea |
Product name: |
HBsAg Rapid Test |
|
Place of Origin: |
Zhejiang, China |
Type: |
Pathological Analysis Equipments |
|
Certificate: |
ISO9001/ISO13485 |
Instrument classification |
Class III |
|
Accuracy: |
99.6% |
Specimen: |
Whole Blood/Serum/Plasma |
|
Format: |
Cassette |
Specification: |
3.00mm/4.00mm |
|
MOQ: |
1000 Pcs |
Shelf life: |
2 years |
|
OEM&ODM |
support |
Specification: |
40pcs/box |
Supply Ability:
5000000 Piece/Pieces per Month
Packaging & delivery:
Packaging Details
40pcs/box
2000PCS/CTN,66*36*56.5cm,18.5KG
Lead time:
| Quantity(pieces) | 1 - 1000 | 1001 - 10000 | >10000 |
| Lead time (days) | 7 | 30 | To be negotiated |
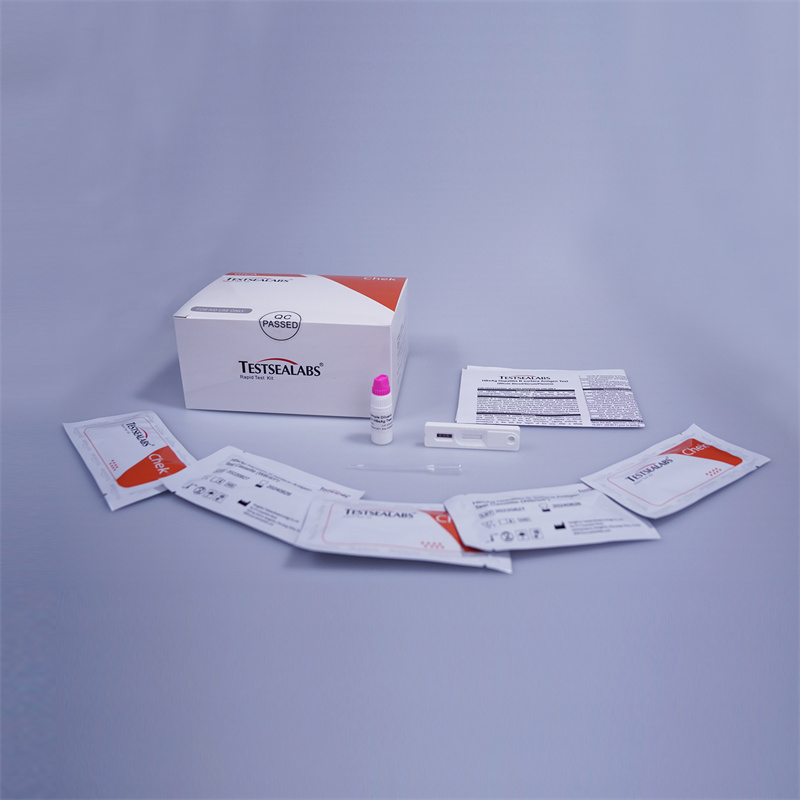
Intended Use
The One Step HBsAg Test is a rapid chromatographic immunoassay for the qualitative detection of Hepatitis B surface Antigen (HBsAg) in Whole Blood /Serum / Plasma.
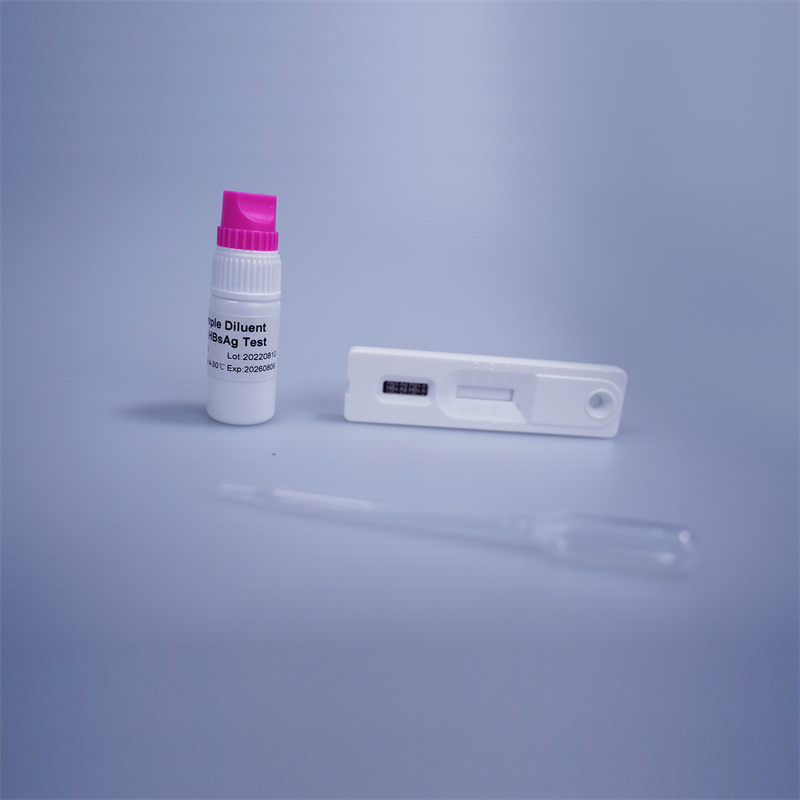
Summary
Hepatitis B is caused by a virus that affects the liver. Adults who get hepatitis B usually recover. However most infants infected at birth become chronic carriers i.e. they carry the virus for many years and can spread the infection to others. The presence of HBsAg in Whole Blood /Serum / Plasma is an indication of an active Hepatitis B infection.
Test Procedure
1. The One Step Test can be performed used on feces.
2. Collect sufficient quantity of feces (1-2 ml or 1-2 g) in a clean, dry specimen collection container to obtain maximum antigens (if present). Best results will be obtained if the assays performed within 6 hours after collection.
3.Specimen collected may be stored for 3 days at 2-8℃ if not tested within 6 hours. For long term storage, specimens should be kept below -20℃.
4.Unscrew the cap of the specimen collection tube, then randomly stab the specimen collection applicator into the fecal specimen in at least 3 different sites to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal of membrane) is not observed in the test window after one minute, add one more drop of specimen to the specimen well.
Positive: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region.
Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure.
★ Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Product List
|
Product name |
Specimen |
Format |
Certificate |
|
Influenza Ag A Test |
Nasal/Nasopharyngeal Swab |
Cassette |
CE ISO |
|
Influenza Ag B Test |
Nasal/Nasopharyngeal Swab |
Cassette |
CE ISO |
|
HCV Hepatitis C Virus Ab Test |
WB/S/P |
Cassette |
ISO |
|
HIV 1+2 Test |
WB/S/P |
Cassette |
ISO |
|
HIV 1/2 Tri-line Test |
WB/S/P |
Cassette |
ISO |
|
HIV 1/2/O Antibody Test |
WB/S/P |
Cassette |
ISO |
|
Dengue IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Dengue NS1 Antigen Test |
WB/S/P |
Cassette |
CE ISO |
|
Dengue IgG/IgM/NS1 Antigen Test |
WB/S/P |
Cassette |
CE ISO |
|
H.Pylori Ab Test |
WB/S/P |
Cassette |
CE ISO |
|
H.Pylori Ag Test |
Feces |
Cassette |
CE ISO |
|
Syphilis(Anti-treponemia Pallidum) Test |
WB/S/P |
Cassette |
CE ISO |
|
Typhoid IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Toxo IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
TB Tuberculosis Test |
WB/S/P |
Cassette |
CE ISO |
|
HBsAg Rapid Test |
WB/S/P |
Cassette |
ISO |
|
HBsAb Rapid Test |
WB/S/P |
Cassette |
ISO |
|
HBeAg Rapid Test |
WB/S/P |
Cassette |
ISO |
|
HBeAb Rapid Test |
WB/S/P |
Cassette |
ISO |
|
HBcAb Rapid Test |
WB/S/P |
Cassette |
ISO |
|
Rotavirus Test |
Feces |
Cassette |
CE ISO |
|
Adenovirus Test |
Feces |
Cassette |
CE ISO |
|
Norovirus Antigen Test |
Feces |
Cassette |
ISO |
|
HAV Hepatitis A virus IgM Test |
WB/S/P |
Cassette |
ISO |
|
HAV Hepatitis A virus IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Malaria Ag p.f/p.v Tri-line Test |
WB |
Cassette |
CE ISO |
|
Malaria Ag p.f/pan Tri-line Test |
WB |
Cassette |
ISO |
|
Malaria Ab p.f/p.v Tri-line Test |
WB |
Cassette |
CE ISO |
|
Malaria Ag p.v Test |
WB |
Cassette |
CE ISO |
|
Malaria Ag p.f Test |
WB |
Cassette |
CE ISO |
|
Malaria Ag pan Test |
WB |
Cassette |
CE ISO |
|
Leishmania IgG/IgM Test |
Serum/Plasma |
Cassette |
CE ISO |
|
Leptospira IgG/IgM Test |
Serum/Plasma |
Cassette |
CE ISO |
|
Brucellosis(Brucella)IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Chikungunya IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Chlamydia trachomatis Ag Test |
Endocervical Swab/Urethral swab |
Cassette |
ISO |
|
Neisseria Gonorrhoeae Ag Test |
Endocervical Swab/Urethral swab |
Cassette |
CE ISO |
|
Chlamydia Pneumoniae Ab IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Chlamydia Pneumoniae Ab IgM Test |
WB/S/P |
Cassette |
ISO |
|
Mycoplasma Pneumonieae Ab IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Mycoplasma Pneumoniae Ab IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Rubella virus Ab IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Cytomegalo virus Antibody IgG/IgM Test |
WB/S/P |
Cassette |
CE ISO |
|
Herpes simplex virus Ⅰ antibody IgG/IgM test |
WB/S/P |
Cassette |
CE ISO |
|
Herpes simplex virus Ⅱ antibody IgG/IgM test |
WB/S/P |
Cassette |
CE ISO |
|
Zika virus antibody IgG/IgM test |
WB/S/P |
Cassette |
CE ISO |
|
Hepatitis E virus antibody IgM test |
WB/S/P |
Cassette |
CE ISO |
|
Influenza Ag A+B Test |
Nasal/Nasopharyngeal Swab |
Cassette |
CE ISO |
|
HCV/HIV/SYP Multi Combo Test |
WB/S/P |
Cassette |
ISO |
|
MCT HBsAg/HCV/HIV Multi Combo Test |
WB/S/P |
Cassette |
ISO |
|
HBsAg/HCV/HIV/SYP Multi Combo Test |
WB/S/P |
Cassette |
ISO |
|
Monkey Pox Antigen Test Cassette |
Oropharyngeal swab |
Cassette |
CE ISO |
Related Products
Exhibition Information






Honorary Certificate

Company Profile


We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
OPACKAGING & SHIPPING
FAQ
We are based in Zhejiang, China, start from 2015,sell to Southeast Asia(15.00%),Domestic Market(15.00%),South,America(10.00%),Africa(10.00%),North America(5.00%),Eastern
Europe(5.00%),Oceania(5.00%),Mid East(5.00%),Eastern Asia(5.00%),Western Europe(5.00%),Central America(5.00%),Northern Europe(5.00%),Southern Europe(5.00%),South Asia(5.00%). There are total about 51-100 people in our office.
Always a pre-production sample before mass production;
Always final Inspection before shipment;
Animals Diagnostic Rapid Test,Fertility Test Kits, Drug of Abuse Test Kits,Infectious Disease Test Kits,Tumor Markers Test,Food Safety Test
Rich strength in technology, advanced equipment, modern management system, comprehensive range of rapid test kits for clinical, family and lab diagnosis, ISO, CE FSC certified
Accepted Delivery Terms: FOB,CIF,EXW,FCA,DDP,Express Delivery;
Accepted Payment Currency: USD;RMB
Accepted Payment Type: T/T, Western Union,Escrow;
Language Spoken: English