Testsea Disease Test Adenovirus Rapid Test Kit
Quick Details
| Brand Name: |
testsea |
Product name: |
Adenovirus Rapid Test Kit
|
|
Place of Origin: |
Zhejiang, China |
Type: |
Pathological Analysis Equipments |
|
Certificate: |
ISO9001/13485 |
Instrument classification |
Class II |
|
Accuracy: |
99.6% |
Specimen: |
Feces |
|
Format: |
Cassete/Strip |
Specification: |
3.00mm/4.00mm |
|
MOQ: |
1000 Pcs |
Shelf life: |
2 years |
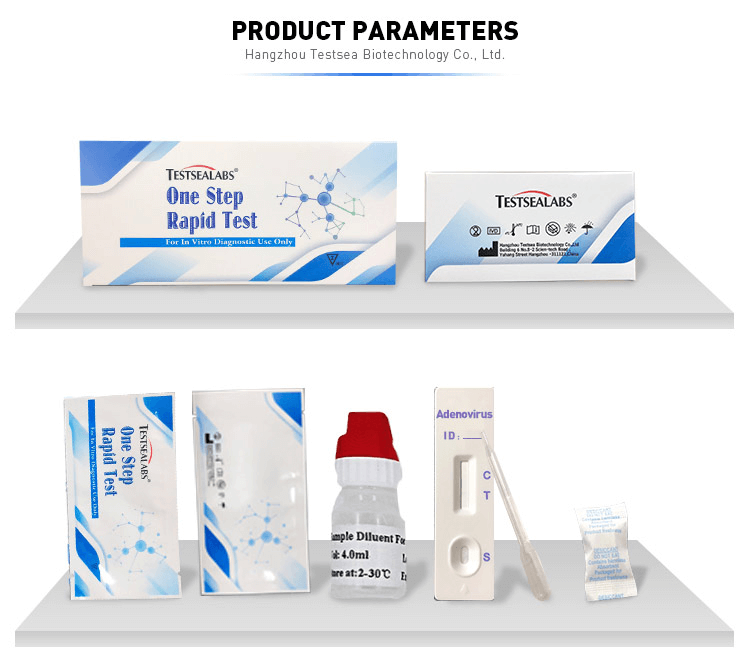
Intended Use
The One Step Adenovirus Test is a qualitative membrane strip based immunoassay for the detection of adenovirus in feces. In this test procedure, Adenovirus antibody is immobilized in the test line region of the device. After an adequate volume of test specimen is placed in the specimen well, it reacts with Adenovirus antibody coated particles that have been applied to the specimen pad. This mixture migrates chromatographically along the length of the test strip and interacts with the immobilized Adenovirus antibody. If the specimen contains Adenovirus, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain Adenovirus, a colored line will not appear in this region indicating a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.

Summary
Adenovirus is the second most common cause of viral gastro-enteritis in Children (10-15%). This virus may also cause respiratory diseases and, depending on the serotype, also diarrhea, conjunctivitis, cystitis, etc. At lease 47 serotypes of adenovirus have been described, all sharing a common hexon antigen. Serotypes 40 and 41 are the ones associated with gastro-enteritis. The main syndrome is diarrhea that may last between 9 and 12 days associated with fever and vomits.
Test Procedure
1. The One Step Test can be performed used on feces.
2. Collect sufficient quantity of feces (1-2 ml or 1-2 g) in a clean, dry specimen collection container to obtain maximum antigens (if present). Best results will be obtained if the assays performed within 6 hours after collection.
3. pecimen collected may be stored for 3 days at 2-8℃ if not tested within 6 hours. For long term storage, specimens should be kept below -20℃.
4. Unscrew the cap of the specimen collection tube, then randomly stab the specimen collection applicator into the fecal specimen in at least 3 different sites to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal of membrane) is not observed in the test window after one minute, add one more drop of specimen to the specimen well.
Positive: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region.
Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure.
★ Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Company Profile
We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
Product Process

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement