Testsealabs Chirwere Chiedza H.Pylori Ag Rapid Test Kit
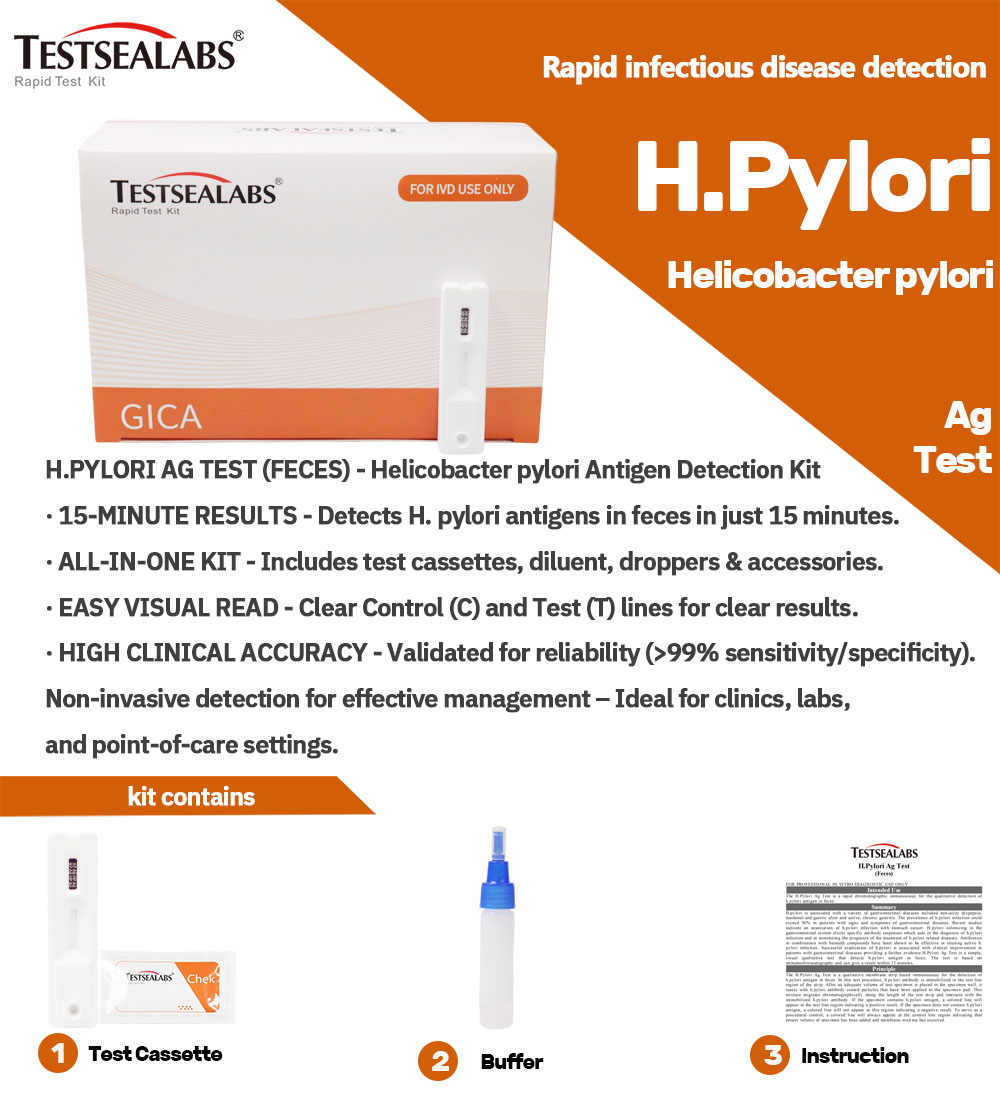
Product Detail:
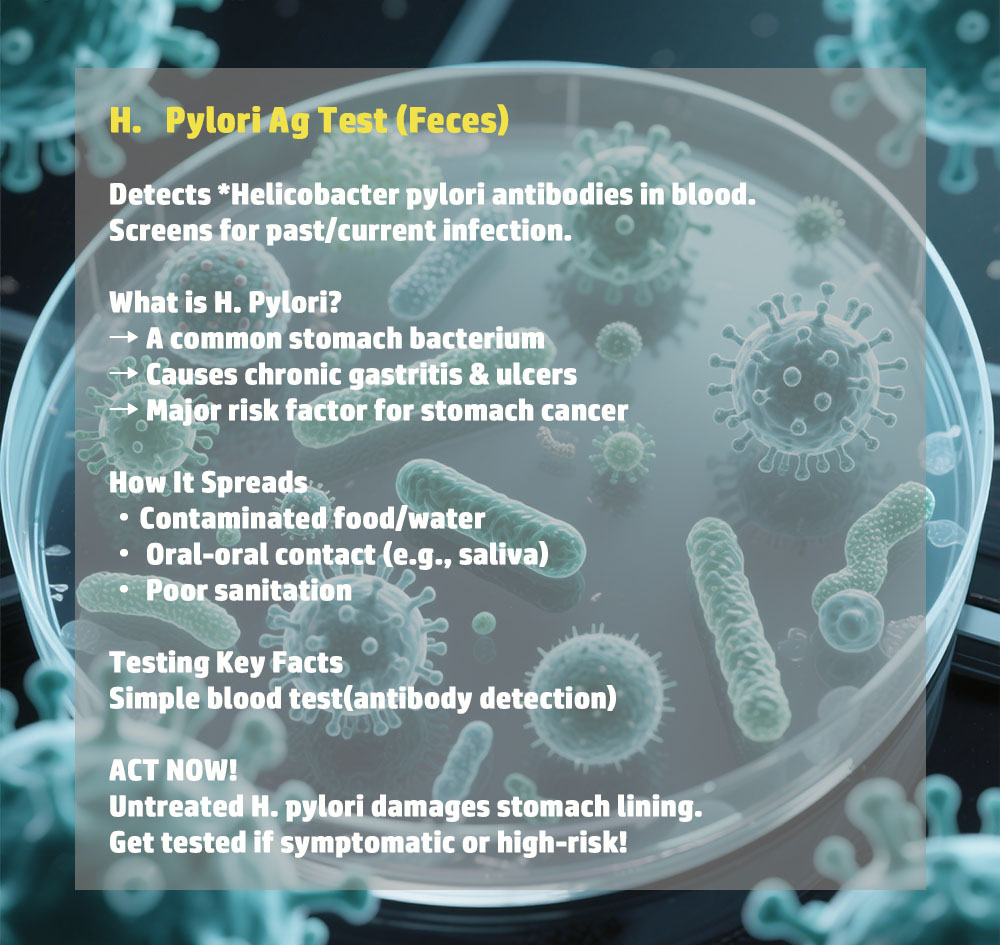
- High Sensitivity uye Kunyatsojeka
Yakagadzirirwa kunyatsoonaH.Pylori Ag Test(tsvina), kupa migumisiro yakavimbika ine ngozi shoma yezvinyorwa zvenhema kana zvisizvo zvenhema. - Rapid Results
Muedzo unopa mhinduro mukatiMaminitsi gumi nemashanu, kufambisa zvisarudzo panguva yakakodzera maererano nehutungamiri hwevarwere uye kutarisira kwekutevera. - Nyore Kushandisa
Muedzo uyu uri nyore kuitisa pasina kudiwa kwehunyanzvi hwekudzidziswa kana zvishandiso, zvichiita kuti ive yakakodzera kushandiswa munzvimbo dzakasiyana dzehutano. - Inotakurika uye Yakanakira Kushandisa Munda
Iyo compact uye lightweight dhizaini yetest kit inoita kuti ive yakakodzeramobile health units, zvirongwa zvekusvika munharaunda,uyemishandirapamwe yehutano hweveruzhinji.
Test Procedure:
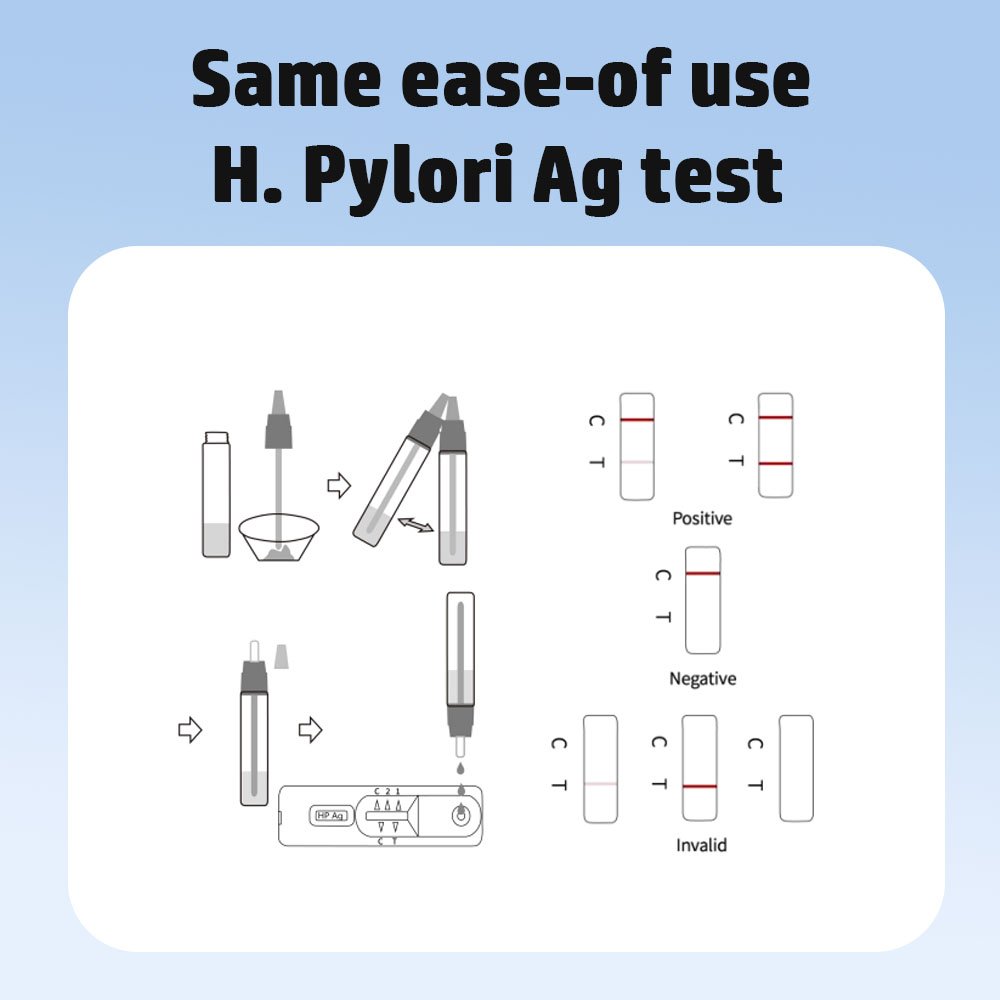
Positive: Mitsetse miviri inooneka. Mutsetse mumwe unofanirwa kugara uchionekwa munharaunda yekudzora mutsara (C), uye imwe mutsara unoonekera weruvara unofanirwa kuoneka munharaunda yebvunzo.
Negative: Mutsara mumwe wemavara unowanikwa munharaunda yekutonga (C) Hapana mutsara unooneka wemavara unowanikwa munharaunda yekuedza.
Hazvina basa: Mutsetse wekudzora unotadza kuoneka. Kusakwana specimen vhoriyamu kana zvisirizvo maitiro emaitiro ndizvo zvinonyanya zvikonzero zvekutadza kwekudzora mutsara. Wongorora maitiro uye dzokorora bvunzo nemudziyo mutsva wekuyedza. Kana dambudziko rikaramba riripo, rega kushandisa test kitnekukasira uye bata muparidzi wenzvimbo yako.








