Testsealabs Monkeypox Virus (MPV) Nucleic Acid Detection Kit
NHUNGAMIDZO
Kiti iyi inoshandiswa pakuongorora in vitro qualitative yevanofungidzirwa kuti vane Monkeypox Virus (MPV), nyaya dzakaungana uye dzimwe nyaya dzinoda kuongororwa hutachiona hweMonkeypox Virus.
Kit inoshandiswa kuona f3L gene yeMPV muhuro swabs uye nasal swab samples.
Mhedzisiro yebvunzo dzekiti iyi ndeyekuongororwa kwekiriniki chete uye haifanirwe kushandiswa seyega mucherechedzo wekuongororwa kwekiriniki. Zvinokurudzirwa kuita kuongororwa kwakazara kwemamiriro ezvinhu zvichienderana nekliniki yemurwere
zviratidzo uye mamwe ma laboratory bvunzo.
Chinangwa Kushandiswa
| Assay type | swabs pahuro uye nasal swab |
| Test type | Qualitative |
| Test material | PCR |
| Pack size | 48 bvunzo / 1 bhokisi |
| Kuchengetedza tembiricha | 2-30 ℃ |
| Sherufu hupenyu | 10 mwedzi |
PRODUCT FEATURE
Principle
Iyi kit inotora iyo chaiyo yakachengetedzwa kutevedzana kweMPV f3L gene senzvimbo inotariswa. Iyo chaiyo-nguva fluorescence quantitative PCR tekinoroji uye nucleic acid nekukurumidza kuburitsa tekinoroji inoshandiswa kutarisa iyo viral nucleic acid kuburikidza neshanduko yefluorescence chiratidzo chezvigadzirwa zveamplification. Iyo yekuona system inosanganisira yemukati yemhando yekudzora, iyo inoshandiswa kutarisa kana kune PCR inhibitors mumasampuli kana kuti masero ari mumasampuri anotorwa, izvo zvinogona kudzivirira zvinobudirira mamiriro enhema asina kunaka.
MAIN COMPONENENT
Iyo kit ine ma reagents ekugadzirisa makumi mana nemasere bvunzo kana kudzora kwemhando, kusanganisira zvinotevera zvikamu:
Reagent A
| Zita | Main components | Quantity |
| Kuonekwa kweMPV reagent | Iyo reaction chubhu ine Mg2+, f3L gene / Rnase P primer probe, reaction buffer, Taq DNA enzyme. | 48 bvunzo |
ReagentB
| Zita | Main components | Quantity |
| MPV Kudzora Kwakanaka | Iine MPV chinangwa chechidimbu | 1 chubhu |
| MPV Negative Control | Pasina MPV chinangwa chidimbu | 1 chubhu |
| DNA kuburitsa reagent | Iyo reagent ine Tris, EDTA uye Triton. | 48pcs |
| Reconstitution reagent | DEPC yakarapwa mvura | 5ML |
Ongorora: Izvo zvikamu zvenhamba dzebatch dzakasiyana hazvigone kushandiswa zvakasiyana
【Storage Mamiriro uye Sherufu Hupenyu】
1.Reagent A / B inogona kuchengetwa pa 2-30 ° C, uye hupenyu hwesherufu ndeye 10 mwedzi.
2.Ndapota vhura test tube cover chete kana wagadzirira bvunzo.
3.Usashandisa test tubes kupfuura zuva rekupera.
4.Usashandisa chubhu yekuona inodonha.
【Applicable Instrument】
Inokodzera Inokodzera LC480 PCR yekuongorora system, Gentier 48E Automatic PCR yekuongorora system, ABI7500 PCR yekuongorora system.
【Mienzaniso Zvinodiwa】
1.Inoshanda mhando dzemhando: huro swabs samples.
2.Sampling mhinduro:Mushure mekuona, zvinokurudzirwa kushandisa yakajairika saline kana Virus kuchengetedza chubhu inogadzirwa neHangzhou Testsea biology yekuunganidza sampuli.
pahuro swab:pukuta bilateral pharyngeal tonsils uye posterior pharyngeal madziro nekuraswa sterile sampling swab, nyudza swab muchubhu ine 3mL sampling solution, kurasa muswe, uye sunga chubhu chivharo.
3.Sample kuchengetedza uye kutumira:Mienzaniso inofanirwa kuongororwa inofanira kuongororwa nekukurumidza sezvinobvira. Kupisa kwekutakura kunofanira kuchengetwa pa 2 ~ 8 ℃. Zvienzaniso zvinogona kuongororwa mukati maawa makumi maviri nemana zvinogona kuchengetwa pa 2 ℃ ~ 8 ℃ uye kana sampuli dzisingagoni kuongororwa mukati maawa makumi maviri nemana, inofanira kuchengetwa zvishoma kudarika kana kuenzana ne -70 ℃ (kana pasina nzvimbo yekuchengetedza ye -70 ℃, inogona kuchengetwa kwenguva pfupi -20 ℃)
kutonhora nekunyunguduka.
4.Kutora sampuli yakakodzera, kuchengetedza, uye kutakura zvakakosha pakushanda kwechigadzirwa ichi.
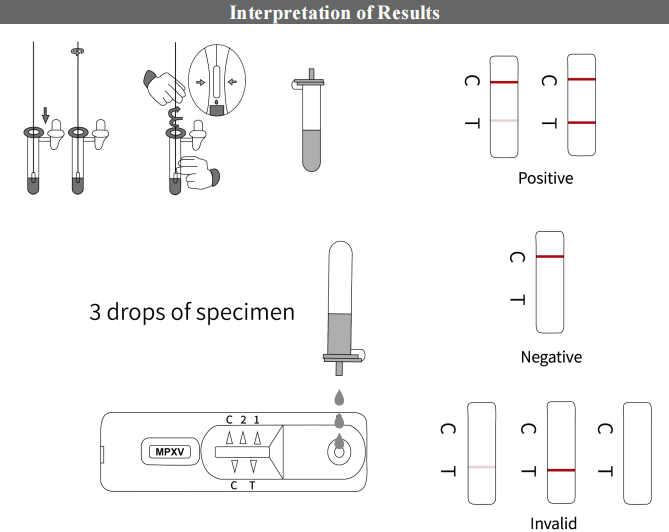
【Testing Method】
1.Sample processing uye sampuli yekuwedzera
1.1 Sample processing
Mushure mekusanganisa mhinduro yesampling iri pamusoro nemasample, tora 30μL yemuenzaniso muDNA inoburitsa reagent chubhu wosanganisa zvakaenzana.
1.2 Loading
Tora 20μL ye reconstitution reagent uye woiwedzera kuMPV yekuona reagent, wedzera 5μL yemuenzaniso wepamusoro wakagadziriswa (Iyo yakanaka yekutonga uye kutonga kusina kunaka ichagadziriswa nenzira yakafanana nemasampuli), kuvhara chubhu, centrifuge iyo pa 2000rpm kwemasekonzi gumi.
2. PCR kuwedzera
2.1 Takura iyo yakagadzirirwa PCR ndiro / machubhu kune fluorescence PCR chiridzwa, Negative control uye kutonga kwakanaka kuchaiswa kune yega bvunzo.
2.2 Fluorescent chiteshi kugadzirisa:
1)Sarudza FAM chiteshi chekuona MPV;
2)Sarudza HEX/VIC chiteshi chemukati chekudzora gene kuona;
3.Kuongorora kwemigumisiro
Seta mutsara wepasi pamusoro penzvimbo yepamusoro yeiyo negative control's fluorescent curve.
4.Quality control
4.1 Negative control: Hapana Ct kukosha yaonekwa muFAM, HEX/VIC chiteshi, kana Ct>40;
4.2 Positive control: MuFAM, HEX/VIC chiteshi, Ct≤40;
4.3 Zvinodiwa zviri pamusoro zvinofanirwa kugutsikana mukuyedza kumwe chete, zvikasadaro mhedzisiro yebvunzo haina basa uye kuyedza kunoda kudzokororwa.
【Bvisa kukosha】
Sample inoonekwa seyakanaka kana: Target sequence Ct≤40, The internal control gene Ct≤40.
【Dudziro yemigumisiro】
Kana kutonga kwemhando kwapfuura, vashandisi vanofanirwa kutarisa kana paine amplification curve yesample yega yega muHEX/VIC chiteshi, kana iripo uye neCt≤40, yakaratidza kuti yemukati yekudzora gene yakakwidziridzwa zvinobudirira uye iyi bvunzo inoshanda. Vashandisi vanogona kuenderera kune yekutevera ongororo:
3.Kumasamples ane kukwidziridzwa kwejene rekutonga kwemukati zvakundikana (HEX/VIC
chiteshi, Ct>40, kana pasina amplification curve), yakaderera Viral load kana kuvepo kwePCR inhibitor kunogona kunge kuri chikonzero chekutadza, bvunzo dzinofanira kudzokororwa kubva mukuunganidzwa kwemuenzaniso;
4.Kune sampuli dzakanaka uye hutachiwana hwakagadzirwa, migumisiro yekutonga kwemukati haibatsiri;
Kune masampuli akaongororwa kuti haana, kutonga kwemukati kunoda kuongororwa kuti ane positive zvikasadaro mhedzisiro yacho haina basa uye bvunzo inoda kudzokororwa, kutanga kubva padanho rekuunganidza.
Exhibition Information






Profile yekambani
Isu, Hangzhou Testsea Biotechnology Co., Ltd ikambani inokura nekukurumidza nyanzvi yebiotechnology nyanzvi mukutsvaga, kugadzira, kugadzira uye kugovera epamberi in-vitro diagnostic (IVD) bvunzo kits uye zviridzwa zvekurapa.
Nzvimbo yedu ndeye GMP, ISO9001, uye ISO13458 certified uye isu tine CE FDA mvumo. Ikozvino tave kutarisira kushanda pamwe nemamwe makambani ari mhiri kwemakungwa mukusimudzirana.
Isu tinogadzira bvunzo dzekubereka, bvunzo dzezvirwere zvinotapukira, bvunzo dzekushandisa zvisina kunaka zvinodhaka, bvunzo dzemwoyo, bvunzo dze tumor marker, chikafu uye chengetedzo bvunzo uye bvunzo dzechirwere chemhuka, uyezve, mhando yedu TESTSEALABS yave ichizivikanwa mumisika yemumba neyekunze. Yakanakisa mhando uye mitengo yakanaka inoita kuti titore pamusoro pe50% migove yemumba.
Product Process

1.Gadzirira

2.Chivharo

3.Muchinjikwa membrane

4.Cheka tambo

5.Assembly

6.Pack the pouches

7.Sima zvikwama

8.Pack bhokisi

9.Encasement

Dzivirira Njodzi Itsva: Gadzirira Izvozvi Sezvo Monkeypox Inopararira
Musi waAugust 14, World Health Organization (WHO) yakazivisa kuti chirwere chemonkeypox chinoumba "Public Health Emergency of International Concern." Aka ndekechipiri WHO ichipa yambiro yepamusoro-soro maererano nekubuda kwemonkeypox kubva muna Chikunguru 2022.
Parizvino, denda remonkeypox rakapararira kubva kuAfrica kuenda kuEurope neAsia, nenyaya dzakasimbiswa dzakataurwa muSweden nePakistan.
Sekureva kwazvino data kubva kuAfrica CDC, gore rino, nyika gumi nembiri dzeAfrican Union dzakataura huwandu hwenyaya 18,737 dzetsoko, kusanganisira 3,101 dzakasimbiswa, 15,636 dzinofungidzirwa, uye 541 vafa, nehuwandu hwekufa kwe2.89%.
01 Chii chinonzi Tsoko?
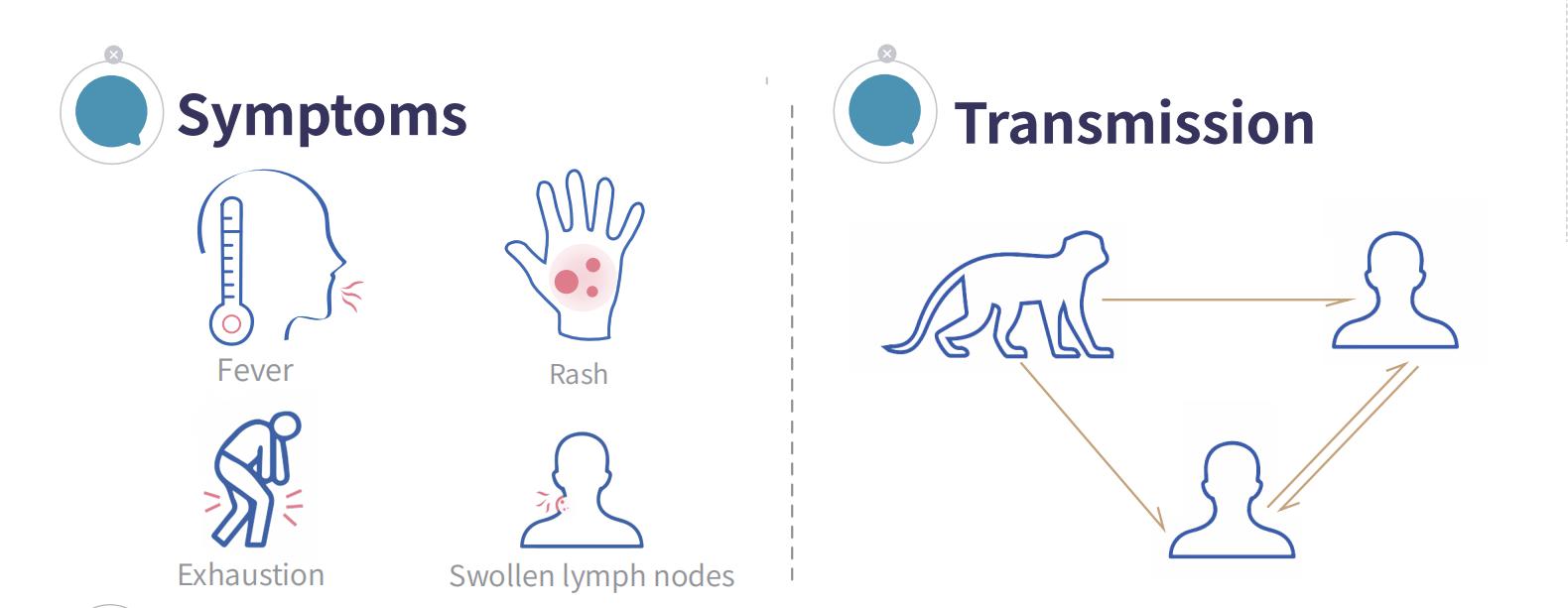
Monkeypox (MPX) chirwere chezoonotic chinokonzerwa nehutachiona hwemonkeypox. Inogona kutapurirwa kubva kumhuka kuenda kuvanhu, uye pakati pevanhu. Zviratidzo zvakajairika zvinosanganisira fivha, rash, uye lymphadenopathy.
Utachiona hwemonkeypox hunonyanya kupinda mumuviri wemunhu kuburikidza ne mucous membranes uye ganda rakatyoka. Nzvimbo dzehutachiona dzinosanganisira zviitiko zvemonkeypox uye makonzo ane hutachiona, tsoko, uye mamwe maprimate asiri vanhu. Mushure mekutapukirwa, incubation nguva ndeyemazuva mashanu kusvika makumi maviri nerimwe, kazhinji mazuva matanhatu kusvika gumi nematatu.
Kunyangwe ruzhinji rwevanhu rwuchibatwa nehutachiona hwemonkeypox, kune imwe nhanho yekudzivirira kubva kutsoko kune avo vakabaiwa nhomba, nekuda kweiyo genetic uye antigenic kufanana pakati pehutachiona. Parizvino, monkeypox inonyanya kupararira pakati pevarume vanoita zvepabonde nevarume kuburikidza nekusangana pabonde, nepo njodzi yekutapukirwa kune ruzhinji inoramba yakaderera.
02 Iyi Monkeypox Kuputika kwakasiyana sei?
Kubva pakutanga kwegore, dambudziko guru rehutachiona hwemonkeypox, "Clade II," rakakonzera kubuda kukuru munyika yose. Zvinoshungurudza, huwandu hwemhosva dzakakonzerwa ne "Clade I," iyo yakanyanya kuoma uye ine huwandu hwekufa kwepamusoro, iri kuwedzera uye yakasimbiswa kunze kwekondinendi yeAfrica. Pamusoro pezvo, kubva munaGunyana gore rapfuura, imwe nyowani, inouraya uye inotapurira nyore nyore, "Clade Ib," yakatanga kupararira muDemocratic Republic of the Congo.
Chiratidzo chechirwere ichi ndechekuti vakadzi nevana vari pasi pemakore gumi nemashanu ndivo vanonyanya kubatwa nechirwere ichi.
Data inoratidza kuti pamusoro pe70% yenyaya dzakataurwa dziri muvarwere vari pasi pemakore gumi nemashanu, uye pakati pezviitiko zvinouraya, nhamba iyi inokwira kusvika 85%. Zvikurukuru,mwero wekufa kwevana wakapetwa kana kupfuura wevakuru.
03 Ndeipi Ngozi yeMonkeypox Transmission?
Nekuda kwemwaka wevashanyi uye kugara uchidyidzana nedzimwe nyika, njodzi yekuyambuka muganhu wehutachiona hwemonkeypox inogona kuwedzera. Nekudaro, hutachiona hunonyanya kupararira kuburikidza nekubatana kwenguva refu, sekusangana pabonde, kubata ganda, uye kufema-pedyo kana kutaura nevamwe, saka kutapurirana kwahwo kumunhu kune-munhu hakuna kusimba.
04 Nzira yekudzivirira Monkeypox?
Dzivisa kusangana pabonde nevanhu vasingazivikanwe hutano hwavo. Vafambi vanofanirwa kutarisisa kubuda kwemonkeypox munyika dzavari kuenda nematunhu uye kudzivirira kusangana nemakonzo nemaprimate.
Kana maitiro ane njodzi huru akaitika, zvitarise hutano hwako kwemazuva makumi maviri nerimwe uye dzivirira kushamwaridzana nevamwe. Kana zviratidzo zvakadai semapundu, mapundu, kana fivha zvikaonekwa, tsvaga kurapwa nekukurumidza uye zivisa chiremba nezvemaitiro akakodzera.
Kana mumwe wemumhuri kana kuti shamwari ikaonekwa kuti ine tsoko, tora matanho ekuzvidzivirira, dzivisa kuswedera pedyo nemurwere, uye usabate zvinhu zvakashandiswa nomurwere, zvakadai sembatya, zvokuvata, matauro, uye zvimwe zvinhu zvomunhu oga. Dzivisa kugoverana zvimbudzi, uye geza maoko kakawanda uye makamuri ekupinza mweya.
Monkeypox Diagnostic Reagents
Monkeypox diagnostic reagents anobatsira kusimbisa hutachiona nekuona mavhairasi maantigen kana masoja ekudzivirira chirwere, achigonesa kuzviparadzanisa nevamwe uye matanho ekurapa, uye kuita basa rakakosha mukudzora zvirwere zvinotapukira. Parizvino, Anhui DeepBlue Medical Technology Co., Ltd. yakagadzira zvinotevera monkeypox diagnostic reagents:
Monkeypox Antigen Test Kit: Inoshandisa colloidal goridhe nzira kuunganidza zvienzaniso zvakaita seoropharyngeal swabs, nasopharyngeal swabs, kana ganda exudates kuti zvionekwe. Inosimbisa hutachiona nekuona kuvapo kwehutachiona hwehutachiona.
Monkeypox Antibody Test Kit: Inoshandisa colloidal goridhe nzira, ine masampuli anosanganisira venous ropa rose, plasma, kana serum. Inosimbisa hutachiona nekuona masoja ekudzivirira chirwere anogadzirwa nemuviri wemunhu kana wemhuka kurwisa hutachiona hwemonkeypox.
Monkeypox Virus Nucleic Acid Test Kit: Inoshandisa chaiyo-nguva fluorescent quantitative PCR nzira, nemuenzaniso uri lesion exudate. Inosimbisa hutachiwana nekuona genome yehutachiona kana kuti magene fragments.
Testsealabs 'Monkeypox Kuedza Zvigadzirwa
Kubva 2015, Testsealabs 'monkeypox diagnostic reagents yakasimbiswa ichishandisa chaiyo hutachiona samples mumarabhoritari ekunze uye dzakapihwa CE Certification nekuda kwekuita kwavo kwakagadzikana uye kwakavimbika. Aya ma reagents anonangana nemhando dzakasiyana dzemuenzaniso, achipa akasiyana masevhisi uye mazinga chaiwo, achipa rutsigiro rwakasimba rwekuonekwa kwehutachiona hwemonkeypox uye nekubatsira zvirinani mukudzora kubuda. Kubva pane rumwe ruzivo nezve yedu monkeypox test kit, ndapota ongorora: https://www.tesselabs.com/monkeypox-virus-mpv-nucleic-acid-detection-kit-product/
Testing maitiro
Kushandisa swab kuunganidza pus kubva pustule, kusanganisa iyo zvakakwana mubuffer, uye wozoisa madonhwe mashoma mukadhi rekuyedza. Mhedzisiro inogona kuwanikwa mumatanho mashoma mashoma.