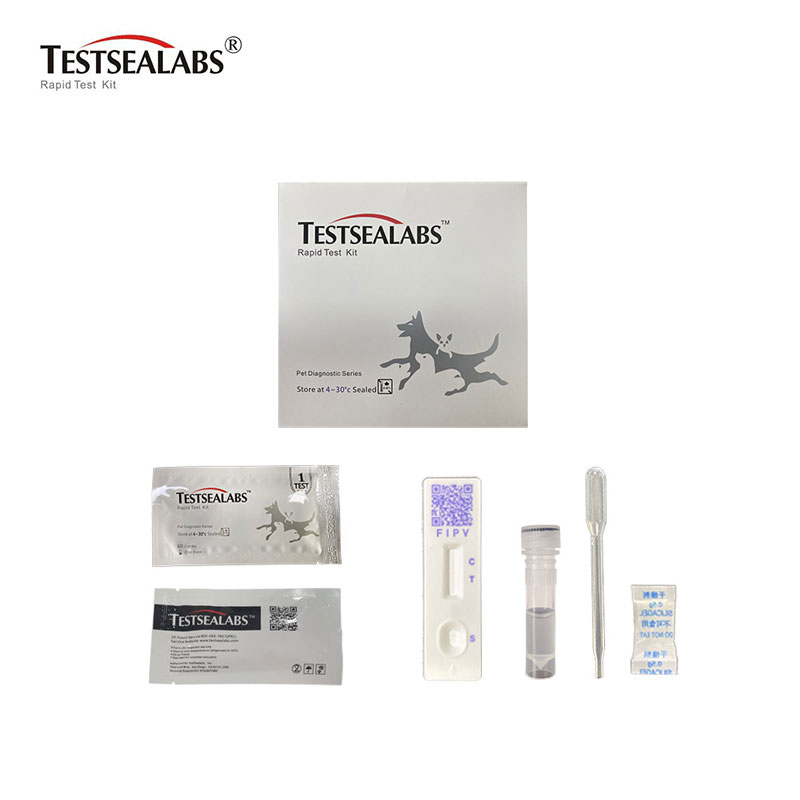
Testsealabs Feline Infectious Peritonitis FIPV RapidTest
Iyo Feline Infectious Peritonitis FIPV Rapid Test bvunzo ine hunyanzvi uye yakanangana nekuonekwa kweFIPV muropa remhuka rakazara kana serum. Muedzo unoburitsa kukurumidza, nyore uye Test mhando pamutengo wakaderera zvakanyanya pane mamwe mabhureki.
Nhanganyaya
| Product Name | FIPV kaseti |
| Brand Name | Testsealabs |
| Place yeOrigin | Hangzhou Zhejiang, China |
| Size | 3.0mm/4.0mm |
| Format | Cassette |
| Muenzaniso | Ropa Rakazara, Serum |
| Kururama | Kupfuura 99% |
| Chitupa | CE/ISO |
| Nguva Yekuverenga | 10min |
| Warranty | Tembiricha yekamuri 24 mwedzi |
| OEM | Available |
Parameter
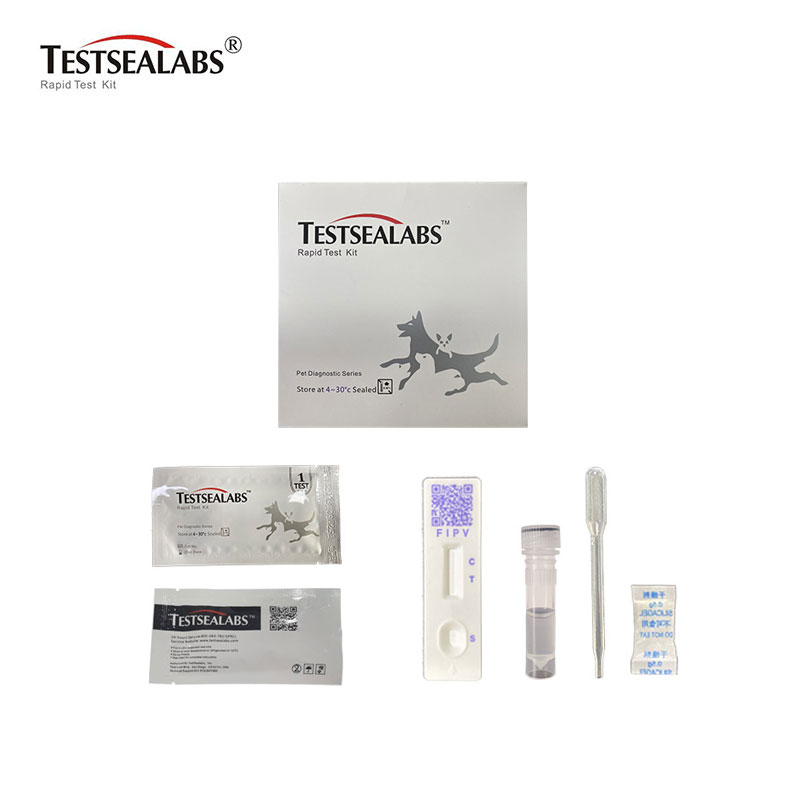
Zvishandiso
• Zvishandiso Zvakagoverwa
1.Test Cassette 2.Droppers 3.Buffer 4.Swap 5.Package Insert
• Zvinhu Zvinodiwa Asi Zvisina Kupiwa
- Timer 2. Magaba ekuunganidza 3.Centrifuge (yeplasma chete) 4.Lancets (yechigunwe cheropa chete) 5.Machubhu eheparinized capillary uye girobhu rekuburitsa (pazvigunwe chete zveropa)
Advantage
| ZVINOGONA ZVAKAVANDUDZWA | Bhodhi rekuona rakakamurwa kuita mitsetse miviri, uye mhedzisiro yacho yakajeka uye iri nyore kuverenga. |
| ZVINYORE | Dzidza kushanda 1 miniti uye hapana midziyo inodiwa. |
| KUDZORERA | 10minutes kunze kwemhedzisiro, hapana chikonzero chekumirira kwenguva refu. |
Nhungamiro Yekushandisa
TEST PROCESS:
1) Bvumira zvese zvikamu zvekiti uye sampuli kuti isvike mukamuri tembiricha isati yaedzwa.
2) Wedzera 1 donho reropa rose, serum kana plasma kune sampuli zvakanaka uye kumirira 30-60seconds.
3) Wedzera 3 madonhwe e buffer kune sampuli tsime.
4) Verenga zvawanikwa mukati me8-10 maminetsi. Usaverenge mushure memaminitsi makumi maviri.
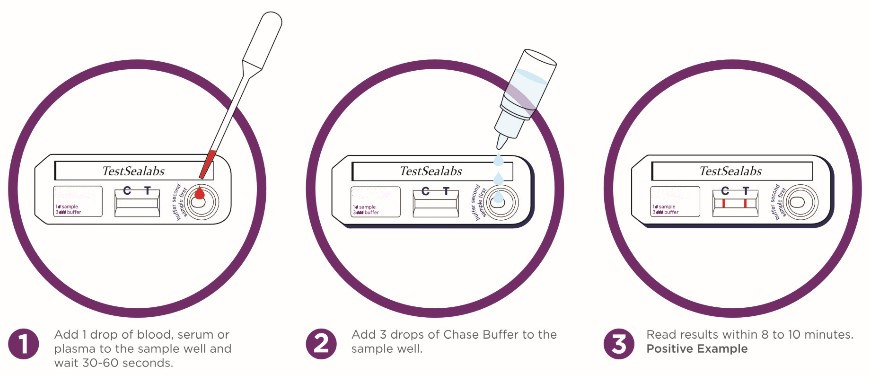
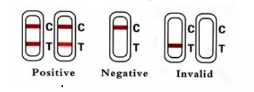
IDUDZIRIRO YEMIZVO
-Zvakanaka (+):Kuvapo kwezvose zviri zviviri "C" mutsara uye zone "T" mutsara, zvisinei T mutsara wakajeka kana kujeka.
-Negative (-):Mutsetse C chete wakajeka unooneka. Hapana mutsara weT.
-Hazvina basa:Hapana mutsara weruvara unoonekwa munzvimbo yeC. Hazvina mhosva kana mutsara weT ukabuda.
Exhibition Information






Profile yekambani
Isu, Hangzhou Testsea Biotechnology Co., Ltd ikambani inokura nekukurumidza nyanzvi yebiotechnology nyanzvi mukutsvaga, kugadzira, kugadzira uye kugovera epamberi in-vitro diagnostic (IVD) bvunzo kits uye zviridzwa zvekurapa.
Nzvimbo yedu ndeye GMP, ISO9001, uye ISO13458 certified uye isu tine CE FDA mvumo. Ikozvino tave kutarisira kushanda pamwe nemamwe makambani ari mhiri kwemakungwa mukusimudzirana.
Isu tinogadzira bvunzo dzekubereka, bvunzo dzezvirwere zvinotapukira, bvunzo dzekushandisa zvisina kunaka zvinodhaka, bvunzo dzemwoyo, bvunzo dze tumor marker, chikafu uye chengetedzo bvunzo uye bvunzo dzechirwere chemhuka, uyezve, mhando yedu TESTSEALABS yave ichizivikanwa mumisika yemumba neyekunze. Yakanakisa mhando uye mitengo yakanaka inoita kuti titore pamusoro pe50% migove yemumba.
Product Process

1.Gadzirira

2.Chivharo

3.Muchinjikwa membrane

4.Cheka tambo

5.Assembly

6.Pack the pouches

7.Sima zvikwama

8.Pack bhokisi

9.Encasement