Testsealabs CEA Carcinoembryonic Antigen Test Kit
Parameter tafura
| Model Number | TIN101 |
| Zita | AFP Alpha-Fetoprotein Test Kit |
| Features | High senitivity, Nyore, Nyore uye Yakarurama |
| Muenzaniso | WB/S/P |
| Tsanangudzo | 3.0mm 4.0mm |
| Kururama | 99.6% |
| Storage | 2'C-30'C |
| Shipping | Negungwa/Nemhepo/TNT/Fedx/DHL |
| Instrument classification | Kirasi II |
| Chitupa | CE ISO FSC |
| Sherufu hupenyu | makore maviri |
| Type | Pathological Analysis Equipments |
Nheyo yeFOB Rapid Test Device
Iyo CEA Rapid Test Device (Ropa Rakazara/Serum/Plasma) yakagadzirirwa kuona munhu carcinoembryonic antigen (CEA) kuburikidza nekududzirwa kwekuona kwekukura kwemavara mukati memutsetse wemukati. Iyo membrane yakanga isingafambiswi ne-anti-CEA kutora masoja ekudzivirira chirwere munzvimbo yekuyedza. Munguva yekuyedzwa, mufananidzo wacho unotenderwa kuita neruvara anti-CEA monoclonal antibodies colloidal goridhe conjugates, iyo yaive yakashongedzwa pane yemuenzaniso pad yebvunzo. Iko kusanganiswa kunobva kwafamba pa membrane ne capillary action, uye kupindirana nema reagents pa membrane. Kana pakanga paine CEA yakakwana mune zvienzaniso, bhendi rine ruvara richaumbwa panzvimbo yekuyedza ye membrane. Kuvapo kwebhandi iri remavara kunoratidza mhedzisiro yakanaka, nepo kusavapo kwayo kunoratidza mhedzisiro yakaipa. Kuonekwa kwebhandi remavara panzvimbo yekutonga inoshanda sekutonga kwemaitiro. Izvi zvinoratidza kuti vhoriyamu yakakodzera yemuenzaniso yakawedzerwa uye membrane wicking yakaitika.

1.Usavhura foil pouch kusvika wagadzirira kutanga kuyedza. Midziyo yekuyedza yefiriji inofanirwa kubvumidzwa kusvika patembiricha yekamuri (15°-28°C) isati yavhura homwe.
2.Bvisa mudziyo kubva muhomwe yekudzivirira uye nyora iyo mudziyo nechiratidzo chekuzivikanwa.
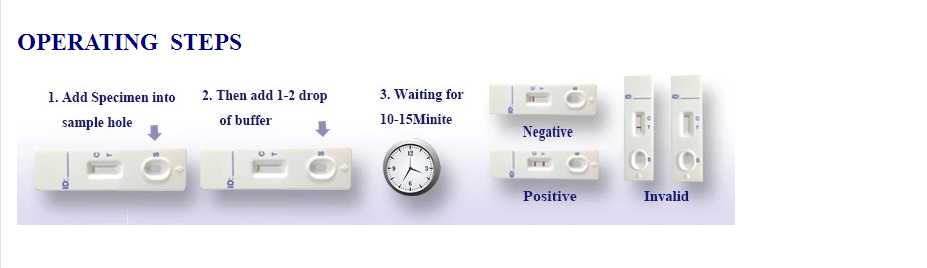
3. Wedzera 50 ul yeropa idzva kuSample Well (yeKadhi) kana Sample Pad (yeDipstick), Wobva wawedzera madonhwe maviri (50 ul) ebvunzo inomhanyisa buffer mutsime remuenzaniso kana sampuli pad.
4. Verenga mhinduro mukati me10- 15 maminitsi. Usaverenge mhinduro mushure memaminitsi gumi nemashanu. Cherechedza
bhandi remavara rakagadzirwa pamusoro penzvimbo yekudzora inoratidza kuti kuyedza kwapera.
Test Procedure
ZVIRI MUKITI
1.Yega yakarongedzerwa bvunzo zvishandiso
Chishandiso chega chega chine tambo ine mavara conjugates uye reactive reagents pre-yakapararira kumatunhu anoenderana.
2.Mapipipi anoraswa
Kuwedzera sampuli shandisa.
3.Buffer
Phosphate yakavharwa nesaline uye inochengetedza.
4.Pakeji isa
Kurairirwa kushanda.
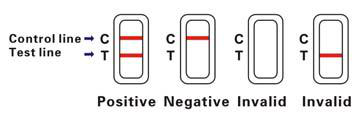
DUDZIRIRO YEMIZVO
Zvakanaka (+)
Mabhendi maviri epingi anooneka panzvimbo yebvunzo. Izvi zvinoratidza kuti mufananidzo wacho une CEA
Negative (-)
Band rimwe chete repink rinoonekwa panzvimbo yebvunzo. Izvi zvinoratidza kuti hapana CEA muropa rose.
Hazvina basa
Kana pasina bhendi remavara richionekwa panzvimbo yebvunzo, ichi chiratidzo chemhosho inogona kuitika pakuita bvunzo. Muedzo unofanirwa kudzokororwa uchishandisa mudziyo mutsva.
Exhibition Information






Profile yekambani
Isu, Hangzhou Testsea Biotechnology Co., Ltd ikambani inokura nekukurumidza nyanzvi yebiotechnology nyanzvi mukutsvaga, kugadzira, kugadzira uye kugovera epamberi in-vitro diagnostic (IVD) bvunzo kits uye zviridzwa zvekurapa.
Nzvimbo yedu ndeye GMP, ISO9001, uye ISO13458 certified uye isu tine CE FDA mvumo. Ikozvino tave kutarisira kushanda pamwe nemamwe makambani ari mhiri kwemakungwa mukusimudzirana.
Isu tinogadzira bvunzo dzekubereka, bvunzo dzezvirwere zvinotapukira, bvunzo dzekushandisa zvisina kunaka zvinodhaka, bvunzo dzemwoyo, bvunzo dze tumor marker, chikafu uye chengetedzo bvunzo uye bvunzo dzechirwere chemhuka, uyezve, mhando yedu TESTSEALABS yave ichizivikanwa mumisika yemumba neyekunze. Yakanakisa mhando uye mitengo yakanaka inoita kuti titore pamusoro pe50% migove yemumba.
Product Process

1.Gadzirira

2.Chivharo

3.Muchinjikwa membrane

4.Cheka tambo

5.Assembly

6.Pack the pouches

7.Sima zvikwama

8.Pack bhokisi

9.Encasement