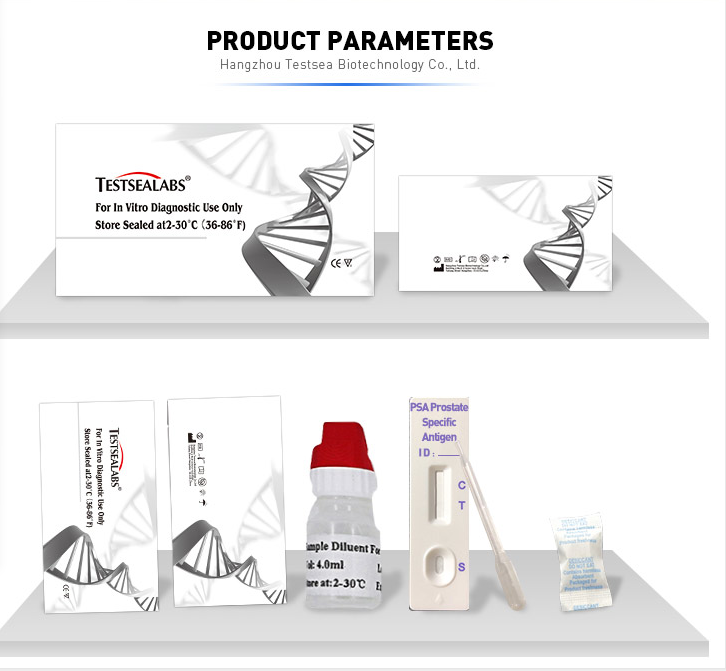
Testsealabs PSA Prostate Specific Antigen Test Kit
Parameter table
| Model Number | TSIN101 |
| Name | PSA Prostate Specific Antigen Qualititive Test Kit |
| Features | High sensitivity, Simple, Easy and Accurate |
| Specimen | WB/S/P |
| Specification | 3.0mm 4.0mm |
| Accuracy | 99.6% |
| Storage | 2'C-30'C |
| Shipping | By sea/By air/TNT/Fedx/DHL |
| Instrument classification | Class II |
| Certificate | CE ISO FSC |
| Shelf life | two years |
| Type | Pathological Analysis Equipments |
Principle of FOB Rapid Test Device
The PSA Rapid Test Device (Whole Blood ) detects prostate specific antigens through visual interpretation of color development on the internal strip. PSA antibodies are immobilized on the test region of the membrane. During testing, the specimen reacts with PSA antibodies conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action, and interacts with reagents on the membrane. If there are sufficient PSA in the specimen, a colored band will form at the test region of the membrane. A test band (T) singal weaker than the reference band (R) indicates that the PSA level in the specimen is between 4-10 ng/mL. A test band (T) signal equal or close to the reference band (R) indicates that the PSA level in the specimen is approximately 10 ng/mL. A test band (T) signal stronger than the reference band (R) indicates that the PSA level in the specimen is above 10 ng/mL. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
The PSA Rapid Test Device (Whole Blood/Serum/Plasma) is a rapid visual immunoassay for the qualitative presumptive detection of prostate specific antigens in human whole blood, serum, or plasma specimens. This kit is intended for use as an aid in the diagnosis of prostate cancer.

Test Procedure
Bring tests, specimens, buffer and/or controls to room temperature before use.
1. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. For best results, the assay should be performed within one hour.
2. Transfer 1 drops of serum/plasma to the specimen well (S) of the device with the provided disposable pipette, then add 1 drop of buffer, and start the timer.
OR
Transfer 2 drops of whole blood to the specimen well (S) of the device with the provided disposable pipette, then add 1 drop of buffer, and start the timer.
OR
Allow 2 hanging drops of fingerstick whole blood to fall into the center of the specimen well (S) of the test device, then add 1 drop of buffer, and start the timer.
Avoid trapping air bubbles in the specimen well (S), and do not add any solution to the result area.
As the test begins to work, color will migrate across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
CONTENT OF THE KIT
The PSA Rapid Test Device (Whole Blood ) is a rapid visual immunoassay for the qualitative presumptive detection of prostate specific antigens in human whole blood, serum, or plasma specimens. This kit is intended for use as an aid in the diagnosis of prostate cancer.
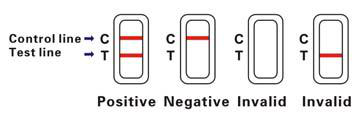
INTERPRETATION OF RESULTS
Positive (+)
Rose-pink bands are visible in both the control region and the test region. It indicates a positive result for hemoglobin antigen.
Negative (-)
A rose-pink band is visible in the control region. No color band appears in the test region. It indicates that the concentration of the hemoglobin antigen is zero or below the detection limit of the test.
Invalid
No visible band at all, or there is a visible band only in the test region but not in the control region. Repeat with a new test kit. If test still fails, please contact the distributor or the store, where you bought the product, with the lot number.
Exhibition Information






Honorary Certificate
Company Profile
We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
Product Process

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement




