OEM/ODM Supplier Oem Service Human Coronavirus Rapid Test - Testsea Disease Test Adenovirus Rapid Test Kit – TESTSEA
OEM/ODM Supplier Oem Service Human Coronavirus Rapid Test - Testsea Disease Test Adenovirus Rapid Test Kit – TESTSEA Detail:
| Brand Name: |
testsea |
Product name: |
Adenovirus Rapid Test Kit
|
|
Place of Origin: |
Zhejiang, China |
Type: |
Pathological Analysis Equipments |
|
Certificate: |
ISO9001/13485 |
Instrument classification |
Class II |
|
Accuracy: |
99.6% |
Specimen: |
Feces |
|
Format: |
Cassete/Strip |
Specification: |
3.00mm/4.00mm |
|
MOQ: |
1000 Pcs |
Shelf life: |
2 years |
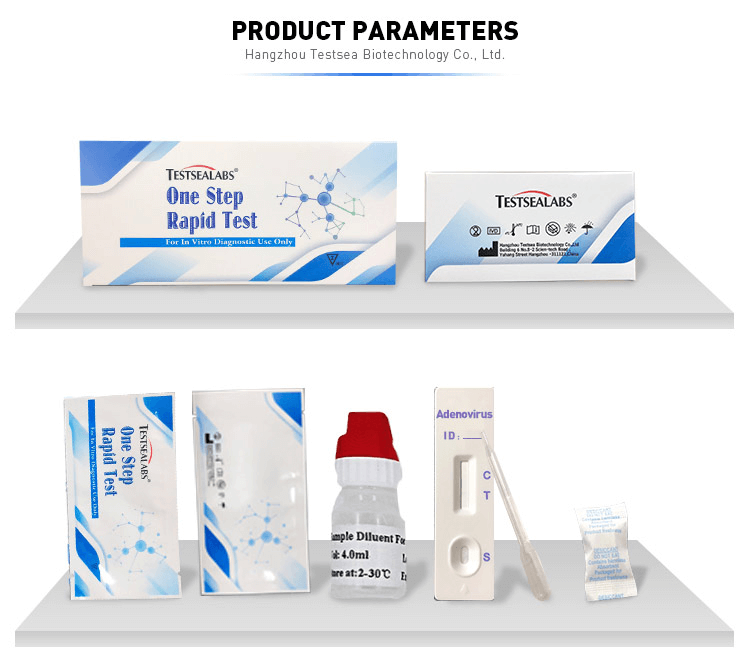
1. The One Step Test can be performed used on feces.
2. Collect sufficient quantity of feces (1-2 ml or 1-2 g) in a clean, dry specimen collection container to obtain maximum antigens (if present). Best results will be obtained if the assays performed within 6 hours after collection.
3. pecimen collected may be stored for 3 days at 2-8℃ if not tested within 6 hours. For long term storage, specimens should be kept below -20℃.
4. Unscrew the cap of the specimen collection tube, then randomly stab the specimen collection applicator into the fecal specimen in at least 3 different sites to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal of membrane) is not observed in the test window after one minute, add one more drop of specimen to the specimen well.
Positive: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region.
Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure.
★ Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.


Product detail pictures:



Related Product Guide:
Our pursuit and company intention is usually to "Always fulfill our purchaser requirements". We go on to acquire and layout excellent high quality products for both our previous and new consumers and realize a win-win prospect for our customers too as us for OEM/ODM Supplier Oem Service Human Coronavirus Rapid Test - Testsea Disease Test Adenovirus Rapid Test Kit – TESTSEA, The product will supply to all over the world, such as: United States, United Arab Emirates, Turin, With more and more Chinese products and solutions around the world, our international business is developing rapidly and economic indicators big increase year by year. We have enough confidence to supply you both better solutions and service, because we've been more and more powerful, specialist and experience in domestic and international.
The customer service staff's attitude is very sincere and the reply is timely and very detailed, this is very helpful for our deal,thank you.
