OEM China Rapid Diagnostic Test - Testsea Disease Test Adenovirus Rapid Test Kit – TESTSEA
OEM China Rapid Diagnostic Test - Testsea Disease Test Adenovirus Rapid Test Kit – TESTSEA Detail:
| Brand Name: |
testsea |
Product name: |
Adenovirus Rapid Test Kit
|
|
Place of Origin: |
Zhejiang, China |
Type: |
Pathological Analysis Equipments |
|
Certificate: |
ISO9001/13485 |
Instrument classification |
Class II |
|
Accuracy: |
99.6% |
Specimen: |
Feces |
|
Format: |
Cassete/Strip |
Specification: |
3.00mm/4.00mm |
|
MOQ: |
1000 Pcs |
Shelf life: |
2 years |
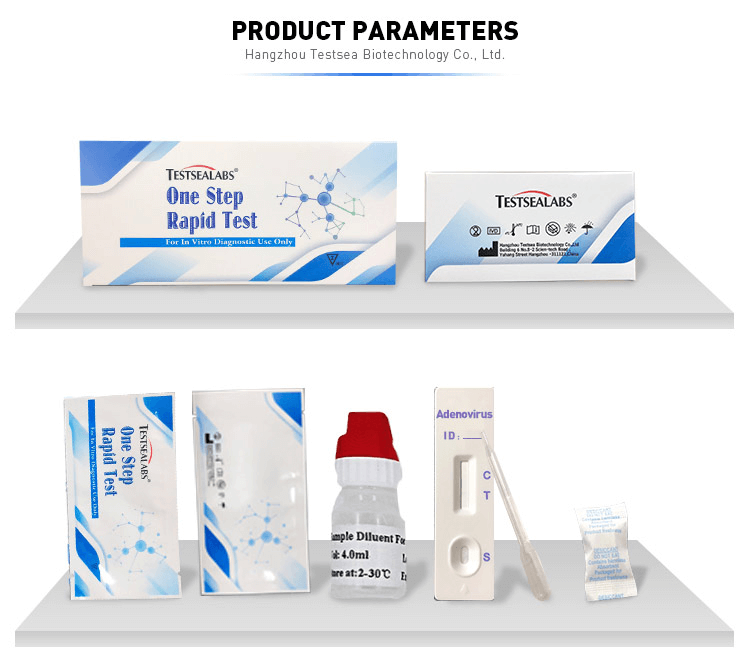
1. The One Step Test can be performed used on feces.
2. Collect sufficient quantity of feces (1-2 ml or 1-2 g) in a clean, dry specimen collection container to obtain maximum antigens (if present). Best results will be obtained if the assays performed within 6 hours after collection.
3. pecimen collected may be stored for 3 days at 2-8℃ if not tested within 6 hours. For long term storage, specimens should be kept below -20℃.
4. Unscrew the cap of the specimen collection tube, then randomly stab the specimen collection applicator into the fecal specimen in at least 3 different sites to collect approximately 50 mg of feces (equivalent to 1/4 of a pea). Do not scoop the fecal of membrane) is not observed in the test window after one minute, add one more drop of specimen to the specimen well.
Positive: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region.
Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure.
★ Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.


Product detail pictures:



Related Product Guide:
Assume full responsibility to meet all demands of our clients; achieve continuous advancements by promoting the growth of our clients; become the final permanent cooperative partner of clients and maximize the interests of clients for OEM China Rapid Diagnostic Test - Testsea Disease Test Adenovirus Rapid Test Kit – TESTSEA, The product will supply to all over the world, such as: Pretoria, Sudan, Bangladesh, Our items have been obtained more and more recognition from foreign clients, and established long term and cooperative relationship with them. We`ll supply the best service for every customer and sincerely welcome friends to work with us and establish the mutual benefit together.
We are really happy to find such a manufacturer that ensuring product quality at the same time the price is very cheap.
