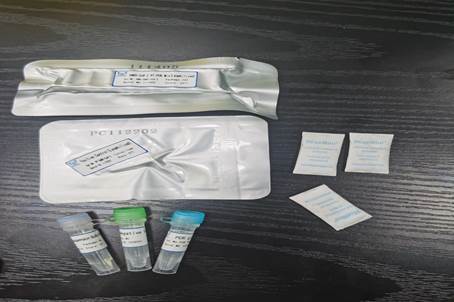
This kit is intended for in vitro qualitative detection of ORF1ab and N genes from the 2019-nCoV in pharyngeal swab or bronchoalveolar lavage specimens collected from Coronavirus disease 2019 (COVID-19) suspected cases, suspected clusters of cases, or other individuals who need 2019- nCoV infection diagnosis or differentiation diagnosis.
The kit is designed for RNA detection of 2019-nCoV in specimens using multiplex real time RTPCR technology and with the conserved regions of ORF1ab and N genes as target sites of the primers and probes. Simultaneously, this kit contains an endogenous control detection system (The control gene is labeled by Cy5) to monitor the process of specimen collection, nucleic acid extraction and PCR and reduce false negative results.
Key Features:
1. Rapid, Reliable amplification and detection inclusivity : SARS like coronavirus and specific detection of SARS-CoV-2
2. One-step RT-PCR reagent (lyophilized powder)
3. Includes positive and negative controls
4. Transportation at normal temperature
5. The kit can keep stable up to 18 months stored at -20℃.
6. CE approved
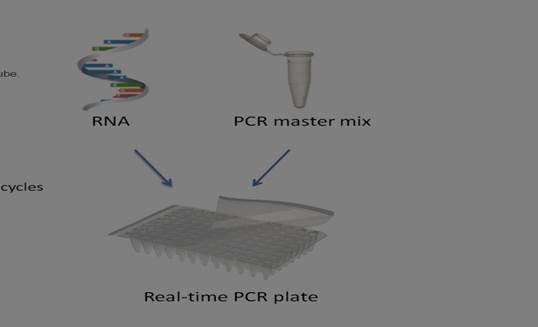
Flow :
1. Prepare extracted RNA from SARS-CoV-2
2. Dilute positive control RNA with water
3. Prepare PCR master mix
4. Apply PCR master mix and RNA into real-time PCR plate or tube
5. Run a real-time PCR instrument
Post time: Nov-09-2020