Monkeypox Virus (MPV) Nucleic Acid Detection Kit
INTRODUCTION
The kit is used for in vitro qualitative detection of suspected cases of Monkeypox Virus (MPV), clustered cases and other cases that need to be diagnosed for Monkeypox Virus infection.
The kit is used to detect the f3L gene of the MPV in throat swabs and nasal swab samples.
The test results of this kit are for clinical reference only and should not be used as the sole criterion for clinical diagnosis. It is recommended to conduct a comprehensive analysis of the condition based on the patient's clinical
manifestations and other laboratory tests.
Intended Use
| Assay type | throat swabs and nasal swab |
| Test type | Qualitative |
| Test material | PCR |
| Pack size | 48tests/1 box |
| Storage temperature | 2-30℃ |
| Shelf life | 10 months |
PRODUCT FEATURE
Principle
This kit takes the specific conserved sequence of the MPV f3L gene as the target region. The real-time fluorescence quantitative PCR technology and nucleic acid rapid release technology are used to monitor the viral nucleic acid through the change of fluorescence signal of amplification products. The detection system includes internal quality control, which is used to monitor whether there are PCR inhibitors in the samples or whether the cells in the samples are taken, which can effectively prevent the false negative situation.
MAIN COMPONENTS
The kit contains reagents for processing 48 tests or quality control, including the following components:
Reagent A
| Name | Main components | Quantity |
| MPV detection
reagent |
The reaction tube contains Mg2+,
f3L gene /Rnase P primer probe, reaction buffer, Taq DNA enzyme. |
48 tests |
Reagent B
| Name | Main components | Quantity |
| MPV
Positive Control |
Containing MPV target fragment | 1 tube |
| MPV
Negative Control |
Without MPV target fragment | 1 tube |
| DNA release reagent | The reagent contains Tris, EDTA
and Triton. |
48pcs |
| Reconstitution reagent | DEPC treated water | 5ML |
Note: The components of different batch numbers cannot be used interchangeably
【Storage Conditions And Shelf Life】
1.Reagent A/B can be stored at 2-30°C, and the shelf life is 10 months.
2.Please open the test tube cover only when you are ready for the test.
3.Do not use test tubes beyond the expiration date.
4.Do not use a leaking detection tube.
【Applicable Instrument】
Suitable for Suitable for LC480 PCR analysis system, Gentier 48E Automatic PCR analysis system, ABI7500 PCR analysis system.
【Sample Requirements】
1.Applicable sample types: throat swabs samples.
2.Sampling solution: After verification, it is recommended to use normal saline or Virus preservation tube produced by Hangzhou Testsea biology for sample collection.
throat swab: wipe bilateral pharyngeal tonsils and posterior pharyngeal wall with disposable sterile sampling swab, immerse the swab into the tube containing 3mL sampling solution, discard the tail, and tighten the tube cover.
3.Sample storage and delivery: The samples to be tested should be tested as soon as possible. The transportation temperature should be kept at 2~8℃.The samples that can be tested within 24 hours can be stored at 2℃~8℃ and if the samples cannot be tested within 24 hours, it should be stored at less than or equal to -70℃ (if there is no storage condition of -70℃, it can be stored at -20℃ temporarily), avoid repeated
freezing and thawing.
4.Proper sample collection, storage, and transportation are critical to the performance of this product.
【Testing Method】
1.Sample processing and sample addition
1.1 Sample processing
After mixing the above sampling solution with samples, take 30μL of the sample into the DNA release reagent tube and mix it evenly.
1.2 Loading
Take 20μL of the reconstitution reagent and add it to the MPV detection reagent, add 5μL of the above processed sample (The positive control and negative control shall be processed in parallel with the samples), cover the tube cap, centrifuge it at 2000rpm for 10 seconds.
2. PCR amplification
2.1 Load the prepared PCR plate/tubes to the fluorescence PCR instrument, Negative control and positive control shall be set for each test.
2.2 Fluorescent channel setting:
1)Choose FAM channel for MPV detection;
2)Choose HEX/VIC channel for internal control gene detection;
3.Results analysis
Set the base line above the highest point of the negative control’s fluorescent curve.
4.Quality control
4.1 Negative control:No Ct value detected in FAM、HEX/VIC channel, or Ct>40;
4.2 Positive control:In FAM、HEX/VIC channel, Ct≤40;
4.3 The above requirements should be satisfied in the same experiment, otherwise the test results are invalid and the experiment needs to be repeated.
【Cut off value】
A sample is considered as positive when: Target sequence Ct≤40, The internal control gene Ct≤40.
【Results interpretation】
Once the quality control is passed, users should check if there is an amplification curve for each sample in HEX/VIC channel, if there is and with Ct≤40, it indicated the internal control gene is successfully amplified and this particular test is valid. Users can proceed to the follow up analysis:
3.For samples with the amplification of internal control gene failed (HEX/VIC
channel, Ct>40,or no amplification curve), low Viral load or the existence of PCR inhibitor could be the reason of failure, the examination should repeated from the specimen collection;
4.For positive samples and cultured virus, the results of internal control do not affect;
For samples tested negative, the internal control needs to be tested positive otherwise the overall result is invalid and the examination needs to be repeated, starting from the specimen collection step
Exhibition Information






Company Profile
We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
Product Process

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement

Prevent a New Tragedy: Prepare Now as Monkeypox Spreads
On August 14th, the World Health Organization (WHO) announced that the monkeypox outbreak constitutes a "Public Health Emergency of International Concern." This is the second time WHO has issued the highest level of alert regarding the monkeypox outbreak since July 2022.
Currently, the monkeypox outbreak has spread from Africa to Europe and Asia, with confirmed cases reported in Sweden and Pakistan.
According to the latest data from the Africa CDC, this year, 12 member states of the African Union have reported a total of 18,737 monkeypox cases, including 3,101 confirmed cases, 15,636 suspected cases, and 541 deaths, with a fatality rate of 2.89%.
01 What is Monkeypox?
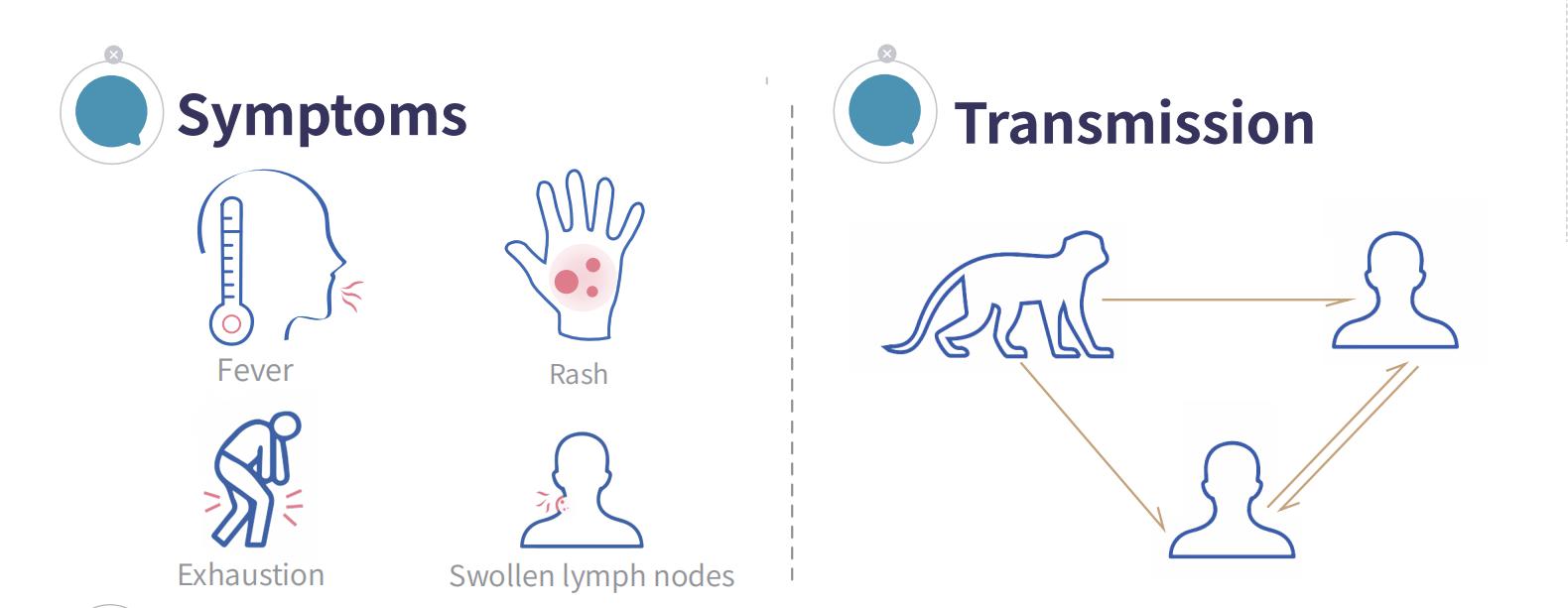
Monkeypox (MPX) is a viral zoonotic disease caused by the monkeypox virus. It can be transmitted from animals to humans, as well as between humans. Typical symptoms include fever, rash, and lymphadenopathy.
The monkeypox virus primarily enters the human body through mucous membranes and broken skin. Sources of infection include monkeypox cases and infected rodents, monkeys, and other non-human primates. After infection, the incubation period is 5 to 21 days, typically 6 to 13 days.
Although the general population is susceptible to the monkeypox virus, there is a certain degree of cross-protection against monkeypox for those who have been vaccinated against smallpox, due to the genetic and antigenic similarities between the viruses. Currently, monkeypox primarily spreads among men who have sex with men through sexual contact, while the risk of infection for the general population remains low.
02 How is This Monkeypox Outbreak Different?
Since the beginning of the year, the main strain of the monkeypox virus, "Clade II," has caused a large-scale outbreak worldwide. Worryingly, the proportion of cases caused by "Clade I," which is more severe and has a higher fatality rate, is increasing and has been confirmed outside the African continent. Additionally, since September last year, a new, more lethal and easily transmissible variant, "Clade Ib," has begun to spread in the Democratic Republic of the Congo.
A notable feature of this outbreak is that women and children under 15 years old are the most affected.
Data shows that over 70% of reported cases are in patients under 15 years old, and among the fatal cases, this figure rises to 85%. Notably, the fatality rate for children is four times higher than for adults.
03 What is the Risk of Monkeypox Transmission?
Due to the tourist season and frequent international interactions, the risk of cross-border transmission of the monkeypox virus may increase. However, the virus mainly spreads through prolonged close contact, such as sexual activity, skin contact, and close-range breathing or talking with others, so its person-to-person transmission capability is relatively weak.
04 How to Prevent Monkeypox?
Avoid sexual contact with individuals whose health status is unknown. Travelers should pay attention to monkeypox outbreaks in their destination countries and regions and avoid contact with rodents and primates.
If high-risk behavior occurs, self-monitor your health for 21 days and avoid close contact with others. If symptoms such as rash, blisters, or fever appear, seek medical attention promptly and inform the doctor of relevant behaviors.
If a family member or friend is diagnosed with monkeypox, take personal protective measures, avoid close contact with the patient, and do not touch items the patient has used, such as clothing, bedding, towels, and other personal items. Avoid sharing bathrooms, and frequently wash hands and ventilate rooms.
Monkeypox Diagnostic Reagents
Monkeypox diagnostic reagents help confirm infection by detecting viral antigens or antibodies, enabling appropriate isolation and treatment measures, and playing an important role in controlling infectious diseases. Currently, Anhui DeepBlue Medical Technology Co., Ltd. has developed the following monkeypox diagnostic reagents:
Monkeypox Antigen Test Kit: Uses colloidal gold method to collect specimens such as oropharyngeal swabs, nasopharyngeal swabs, or skin exudates for detection. It confirms infection by detecting the presence of viral antigens.
Monkeypox Antibody Test Kit: Uses colloidal gold method, with samples including venous whole blood, plasma, or serum. It confirms infection by detecting antibodies produced by the human or animal body against the monkeypox virus.
Monkeypox Virus Nucleic Acid Test Kit: Uses real-time fluorescent quantitative PCR method, with the sample being lesion exudate. It confirms infection by detecting the virus's genome or specific gene fragments.
Testsealabs’ Monkeypox Testing Products
Since 2015, Testsealabs’ monkeypox diagnostic reagents have been validated using real virus samples in foreign laboratories and have been CE certified due to their stable and reliable performance. These reagents target different sample types, offering various sensitivity and specificity levels, providing strong support for monkeypox infection detection and better assisting in effective outbreak control. Fro more information about our monkeypox test kit, please review: https://www.testsealabs.com/monkeypox-virus-mpv-nucleic-acid-detection-kit-product/
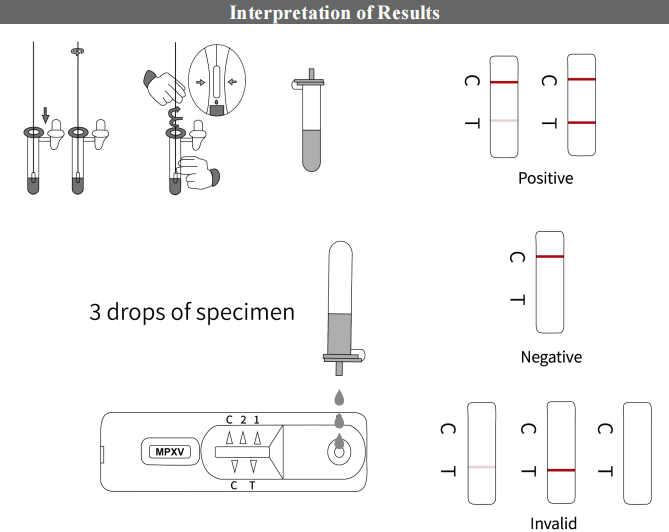
Testing procedure
Using a swab to collect pus from the pustule, mixing it thoroughly in the buffer, and then applying a few drops into the test card. The result can be obtained in just a few simple steps.