MonkeyPox Antigen Test Cassette (Serum/Plasma/Swabs)
Product Detail:
- High Sensitivity and Specificity
The test is designed to provide accurate detection of Monkeypox virus antigens or antibodies, with minimal cross-reactivity with other similar viruses. - Rapid Results
Results are available within 15-20 minutes, making it ideal for quick decision-making in clinical settings or during outbreaks. - Ease of Use
The test is user-friendly and requires no specialized training or equipment. It is suitable for use by healthcare professionals in various settings, including emergency rooms, outpatient clinics, and field hospitals. - Versatile Sample Types
The test is compatible with whole blood, serum, or plasma, offering flexibility in sample collection. - Portable and Ideal for Field Use
The compact design of the test makes it ideal for use in mobile health units, community outreach programs, and epidemic response situations.
Principle:
The Monkeypox Rapid Test Kit works on the principle of lateral flow immunochromatography, where the test detects either Monkeypox virus antigens or antibodies. The process is as follows:
- Sample Collection
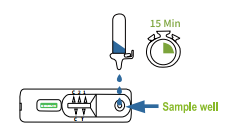
A small volume of whole blood, serum, or plasma is added to the sample well of the test device. A buffer solution is then applied to facilitate the flow of the sample. - Antigen-Antibody Reaction
The test cassette contains recombinant antigens or antibodies specific to the Monkeypox virus. If the sample contains Monkeypox virus-specific antibodies (IgM, IgG) or antigens from an active infection, they will bind to the corresponding component on the test strip. - Chromatographic Migration
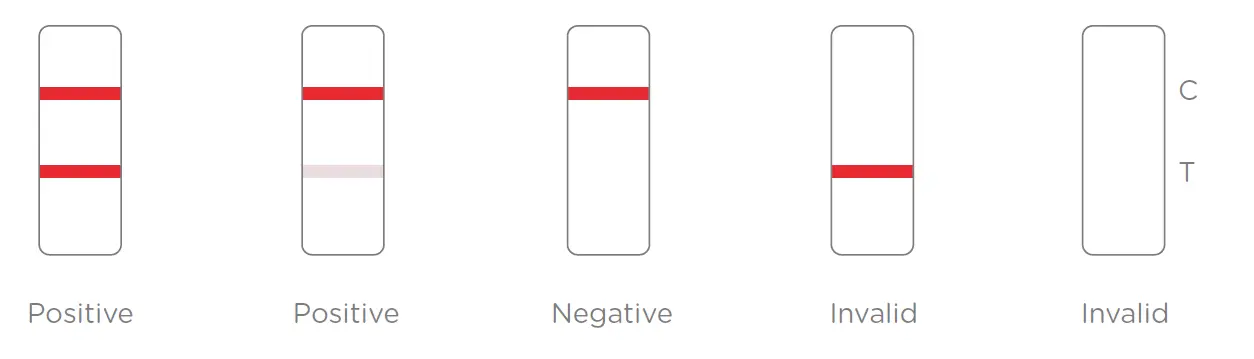
The sample moves along the membrane due to capillary action. If the Monkeypox-specific antigens or antibodies are present, they will bind to the test line (T line), producing a visible colored band. The movement of reagents also ensures the formation of a control line (C line), which confirms the validity of the test. - Result Interpretation
- Two lines (T line + C line): Positive result, indicating the presence of Monkeypox virus antigen or antibodies.
- One line (C line only): Negative result, indicating no detectable Monkeypox virus antigen or antibodies.
- No line or T line only: Invalid result, requiring a retest.
Composition:
|
Composition |
Amount |
Specification |
|
IFU |
1 |
/ |
|
Test cassette |
25 |
Each sealed foil pouch containing one test device and one desiccant |
|
Extraction diluent |
500μL*1 Tube *25 |
Tris-Cl buffer, NaCl, NP 40, ProClin 300 |
|
Dropper tip |
/ |
/ |
|
Swab |
25 |
/ |
Test Procedure:
|
|
|
|
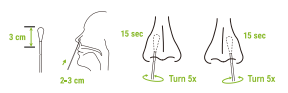
5.Carefully remove the swab without touching the tip.Insert the entire tip of the swab 2 to 3 cm into the right nostril.Note the breaking point of the nasal swab.You can feel this with your fingers when inserting the nasal swab or check it in the mimnor. Rub the inside of the nostril in circular movements 5 times for at least 15 seconds,Now take the same nasal swab and insert it into the other nostril.Swab the inside of the nostril in a circular motion 5 times for at least 15 seconds. Please perform the test directly with the sample and do not
|
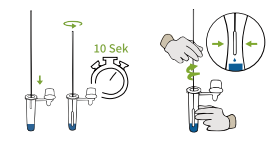
6.Place the swab in the extraction tube.Rotate the swab for about 10 seconds,Rotate the swab against the extraction tube,pressing the head of the swab against the inside of the tube while squeezing the sides of the tube to release as much liquid as possible from the swab. |
|
7. Take out the swab from the package without touching the padding. |
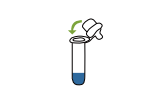
8.Mix thoroughly by flicking the bottom of the tube.Place 3 drops of the sample vertically into the sample well of the test cassette.Read the result after 15 minutes. Note:Read the result within 20 minutes.Otherwise,are petition of the test is recommended. |
Results Interpretation: