Examen Morbi Testsealabs Syphilis (Anti-Treponemiae Pallidae)
Breves Notitiae
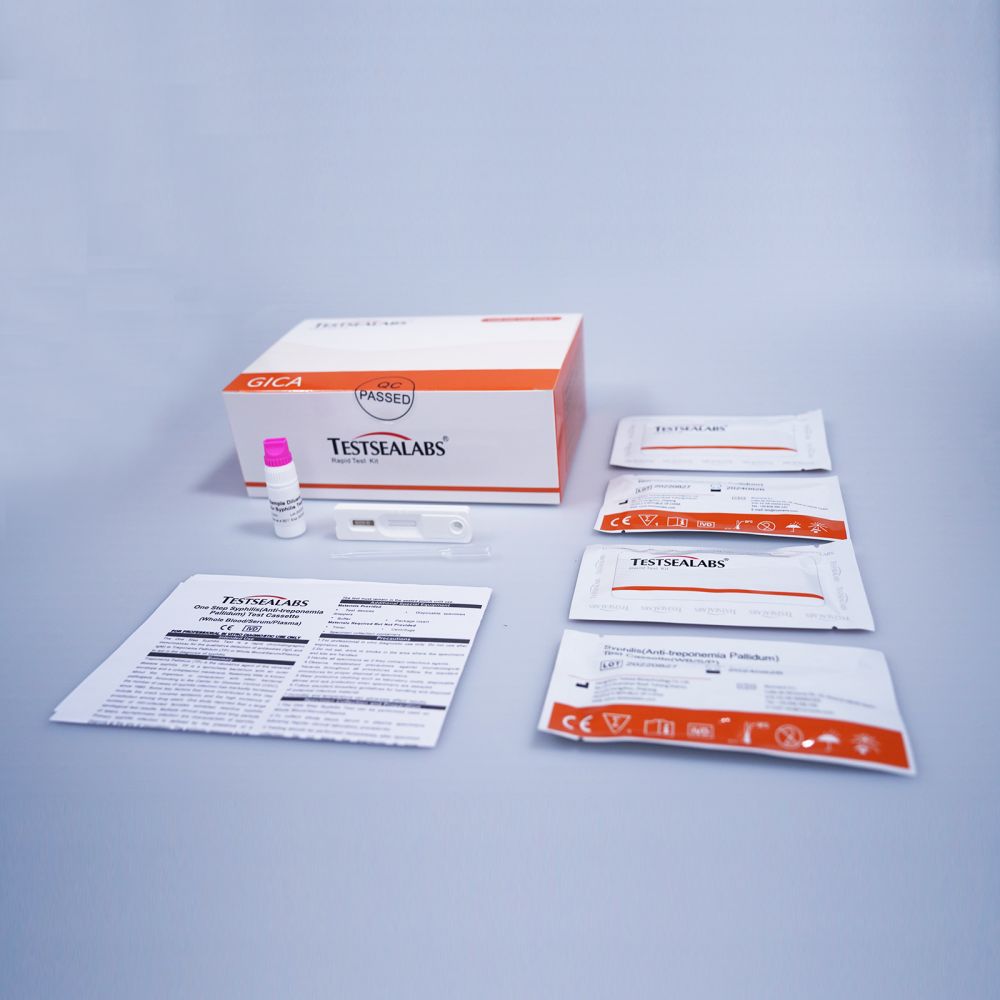
| Nomen Marcae: | Mare Probationis | Nomen producti: | Syphilis (Anti-treponemia Pallidum) Test |
| Locus Originis: | Zhejiang, China | Typus: | Instrumenta Analyseos Pathologicae |
| Certificatum: | CE/ISO9001/ISO13485 | Classificatio instrumentorum | Classis III |
| Accuratio: | 99.6% | Specimen: | Sanguis Totus/Serum/Plasma |
| Forma: | Cassetta | Specificatio: | 3.00mm/4.00mm |
| Quantitas minima (MOQ): | Mille frusta | Tempus conservationis: | Biennium |
| OEM et ODM | auxilium | Specificatio: | Quadraginta partes/capsa |
Facultatem Copiae:
5000000 frustum/frustula per mensem
Involucrum et traditio:
Detalia Involucri
Quadraginta partes/capsa
2000 partes/CTN, 66*36*56.5cm, 18.5kg
Tempus ductionis:
| Quantitas (partes) | 1 - 1000 | 1001 - 10000 | >10000 |
| Tempus executionis (dies) | 7 | 30 | Ad negotiandum |
Descriptio Videonis
Usus Destinatus
Cassetta Probationis Celeris Anticorporum Syphilis (SYP) (Sanguis Totus/Serum/Plasma) est experimentum rapidum, serologicum, immunochromatographicum ad detectionem qualitativam anticorporum (IgG, IgM et IgA) ad Treponema Pallidum (TP) in sero vel plasma humano. Destinata est ad usum ut experimentum perscrutatorium et ut auxilium in diagnosi infectionis cum TP. Quodvis specimen reactivum cum cassetta probationis celeris SYP Ab confirmandum est cum methodo(ibus) probationis alternativa(is) et inventis clinicis.
Summarium
Treponema pallidum (TP) est agens causativus syphilis, morbi venerei. TP est bacterium spirochetum cum involucro externo et membrana cytoplasmatica. Relative parum de organismo notum est, comparatione cum aliis pathogenis bacterialibus. Secundum Centrum pro Morborum Imperio (CDC), numerus casuum infectionis syphilis insigniter auctus est ab anno 1985. Inter factores clavis qui ad hunc incrementum contulerunt sunt epidemia cocaini crack et magna incidentia prostitutionis inter usores medicamentorum. Unum studium rettulit magnum numerum feminarum HIV infectarum eventus probationum serologicarum syphilis reactivarum ostendisse. Stadia clinica multiplicia et longa tempora infectionis latentis, asymptomaticae syphilis propria sunt. Infectio syphilis primaria definitur praesentia chancri in loco inoculationis. Responsio anticorporum ad bacterium TP detegi potest intra 4 ad 7 dies postquam chancrus apparet. Infectio manet detectabilis donec aegrotus curationem idoneam accipit.
Ratio Probationis
1. Examen Unius Gradus in faecibus fieri potest.
2. Satis magnam quantitatem faecum (1-2 ml vel 1-2 g) in vase mundo et sicco ad specimina colligenda collige ut maxima antigena (si adsint) obtineantur. Optimi eventus obtinebuntur si probationes intra 6 horas post collectionem perficiuntur.
3. Specimina collecta, nisi intra sex horas probentur, per tres dies ad 2-8°C conservari possunt. Ad diuturnam conservationem, specimina infra -20°C conservanda sunt.
4. Operculum tubi collectionis speciminis solve, deinde applicatorem collectionis speciminis temere in specimen faecale in saltem tribus locis diversis punge ut circiter 50 mg faecalis (aequivalens 1/4 pisorum) colligas. Ne faeces ex membrana cochleari curras; si in fenestra probationis post unum minutum non observatur, guttam speciminis in puteum speciminis adde.
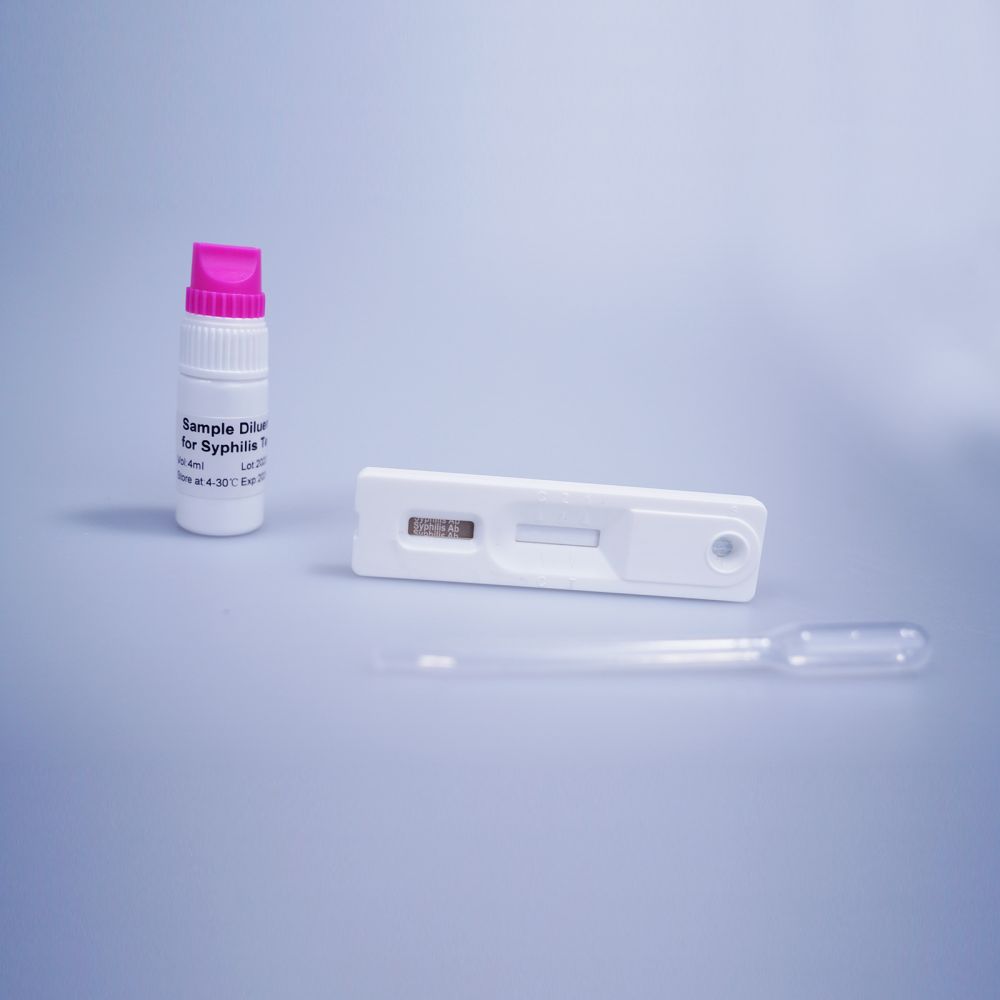
Positivum: Duae lineae apparent. Una linea semper in regione lineae moderatoriae (C) apparere debet, et altera linea colorata manifesta in regione lineae probationis apparere debet.
Negativum: Una linea colorata in regione moderatrice (C) apparet. Nulla linea colorata manifesta in regione lineae probationis apparet.
Invalidum: Linea moderatoria non apparet. Volumen speciminis insufficiens vel rationes procedurales incorrectae sunt causae probabilissimae defectus lineae moderatoriae.
★ Rationem perlege et experimentum cum novo instrumento itera. Si problema perseverat, statim instrumentum experimenti uti desine et distributorem localem contange.
Index Productorum
| Nomen producti | Specimen | Forma | Certificatum |
| Examen Influenzae Ag A | Peniculus Nasalis/Nasopharyngeus | Cassetta | CE ISO |
| Examen Influenzae Ag B | Peniculus Nasalis/Nasopharyngeus | Cassetta | CE ISO |
| Examen Anticorporum Virus Hepatitis C HCV | WB/S/P | Cassetta | ISO |
| Examen HIV 1+2 | WB/S/P | Cassetta | ISO |
| Examen Tri-lineare HIV 1/2 | WB/S/P | Cassetta | ISO |
| Examen Anticorporum HIV 1/2/O | WB/S/P | Cassetta | ISO |
| Examen IgG/IgM Dengue | WB/S/P | Cassetta | CE ISO |
| Examen Antigeni NS1 Dengue | WB/S/P | Cassetta | CE ISO |
| Examen Antigeni IgG/IgM/NS1 Dengue | WB/S/P | Cassetta | CE ISO |
| Examen anticorporum H. Pylori | WB/S/P | Cassetta | CE ISO |
| Examen H. Pylori Ag | Faeces | Cassetta | CE ISO |
| Syphilis (Anti-treponemia Pallidum) Test | WB/S/P | Cassetta | CE ISO |
| Examen IgG/IgM Typhoideum | WB/S/P | Cassetta | CE ISO |
| Examen Toxo IgG/IgM | WB/S/P | Cassetta | CE ISO |
| Examen Tuberculosis Tuberculosis | WB/S/P | Cassetta | CE ISO |
| Examen Celere HBsAg | WB/S/P | Cassetta | ISO |
| Examen Celere HBsAb | WB/S/P | Cassetta | ISO |
| Examen Celere HBeAg | WB/S/P | Cassetta | ISO |
| Examen Celere HBeAb | WB/S/P | Cassetta | ISO |
| Examen Celere HBcAb | WB/S/P | Cassetta | ISO |
| Examen Rotavirus | Faeces | Cassetta | CE ISO |
| Examen Adenovirale | Faeces | Cassetta | CE ISO |
| Examen Antigeni Noroviralis | Faeces | Cassetta | ISO |
| Examen IgM virusis hepatitis A (HAV) | WB/S/P | Cassetta | ISO |
| Examen IgG/IgM virusis hepatitis A (HAV) | WB/S/P | Cassetta | CE ISO |
| Examen Tri-lineare pf/pv Malariae Ag | WB | Cassetta | CE ISO |
| Examen Tri-lineare Malariae Ag pf/pan | WB | Cassetta | ISO |
| Examen Tri-lineare pf/pv Anti-Malariae | WB | Cassetta | CE ISO |
| Examen malariae Ag pv | WB | Cassetta | CE ISO |
| Examen Malariae Ag pf | WB | Cassetta | CE ISO |
| Examen Ag pan malariae | WB | Cassetta | CE ISO |
| Examen IgG/IgM Leishmaniae | Serum/Plasma | Cassetta | CE ISO |
| Examen IgG/IgM Leptospirae | Serum/Plasma | Cassetta | CE ISO |
| Examen IgG/IgM Brucellosis (Brucellae) | WB/S/P | Cassetta | CE ISO |
| Examen IgM Chikungunyae | WB/S/P | Cassetta | CE ISO |
| Examen Ag Chlamydiae trachomatis | Peniculus Endocervical/Peniculus Urethralis | Cassetta | ISO |
| Examen Neisseriae Gonorrhoeae Ag | Peniculus Endocervical/Peniculus Urethralis | Cassetta | CE ISO |
| Examen IgG/IgM ab Chlamydiae Pneumoniae | WB/S/P | Cassetta | CE ISO |
| Examen Anticorporum IgM pro Chlamydia Pneumoniae | WB/S/P | Cassetta | ISO |
| Examen IgG/IgM contra Mycoplasma Pneumoniae | WB/S/P | Cassetta | CE ISO |
| Examen Mycoplasmatis Pneumoniae Ab IgM | WB/S/P | Cassetta | CE ISO |
| Examen IgG/IgM ab virus rubellae | WB/S/P | Cassetta | CE ISO |
| Examen Anticorporum IgG/IgM Virus Cytomegalo | WB/S/P | Cassetta | CE ISO |
| Examen anticorporum IgG/IgM ad virus herpes simplex II | WB/S/P | Cassetta | CE ISO |
| Examen anticorporum IgG/IgM ad virus herpes simplex II | WB/S/P | Cassetta | CE ISO |
| Examen IgG/IgM anticorporis virus Zika | WB/S/P | Cassetta | CE ISO |
| Examen IgM anticorporum virus hepatitidis E | WB/S/P | Cassetta | CE ISO |
| Examen Influenzae Ag A+B | Peniculus Nasalis/Nasopharyngeus | Cassetta | CE ISO |
| Examen Multiplex Compositum HCV/HIV/SYP | WB/S/P | Cassetta | ISO |
| Examen MCT HBsAg/HCV/HIV Multi Compositum | WB/S/P | Cassetta | ISO |
| Examen Multiplex Compositum HBsAg/HCV/HIV/SYP | WB/S/P | Cassetta | ISO |
| Cassetta Examinis Antigeni Variolae Simiae | Peniculus oropharyngeus | Cassetta | CE ISO |
Informationes Exhibitionis






Diploma Honorarium

Descriptio Societatis


Nos, Hangzhou Testsea Biotechnology Co., Ltd, est societas biotechnologica professionalis celeriter crescens, specializata in investigatione, evolutione, fabricatione et distributione apparatuum probationum diagnosticarum in vitro (IVD) et instrumentorum medicorum provectorum.
Officina nostra certificationibus GMP, ISO9001, et ISO13458 praedita est, et approbatione CE FDA fruimur. Nunc cum pluribus societatibus transmarinis cooperationem ad mutuum progressum exspectamus.
Examina fertilitatis, morborum contagiosorum, abusus medicamentorum, signorum cardiacorum, signorum tumorum, ciborum et salutis, et morborum animalium producimus. Praeterea, nostra marca TESTSEALABS tam in mercatibus domesticis quam transmarinis bene nota est. Optima qualitas et pretia commoda nobis permittunt ut plus quam dimidiam partem partium domesticarum obtineamus.
OINVOLUCRUM ET TRANSPORTATIO
Quaestiones Frequentes
In Zhejiang, Sinis, sedes habemus; ab anno 2015 incipimus, et ad Asiam Meridionalem-Orientalem (15.00%), Mercatum Domesticum (15.00%), Meridianam, Americam (10.00%), Africam (10.00%), Americam Septentrionalem (5.00%), Orientalem vendimus.
Europa (5.00%), Oceania (5.00%), Oriens Medius (5.00%), Asia Orientalis (5.00%), Europa Occidentalis (5.00%), America Centralis (5.00%), Europa Septentrionalis (5.00%), Europa Meridionalis (5.00%), Asia Meridionalis (5.00%). Sunt in summa circiter 51-100 homines in officio nostro.
Semper exemplum prae-productionis ante productionem massalem;
Semper inspectio finalis ante vecturam;
Examen Diagnosticum Celere Animalium, Instrumenta Probationis Fertilitatis, Instrumenta Probationis Medicamentorum Abusus, Instrumenta Probationis Morborum Infectiosorum, Examen Signorum Tumoris, Examen Salutis Cibi
Dives in technologia, apparatu provecto, systemate administrationis moderno, series completa instrumentorum probationum celerium ad diagnosin clinicam, familiarem et laboratorium, certificatione ISO, CE et FSC.
Conditiones traditionis acceptae: FOB, CIF, EXW, FCA, DDP, Express Tradition;
Pecunia Solutionis Accepta: USD;RMB
Genus Solutionis Acceptum: T/T, Western Union, Escrow;
Lingua Adhibita: Anglica
