Instrumentum celerem ad morbum HCV Ab examinandum Testsealabs
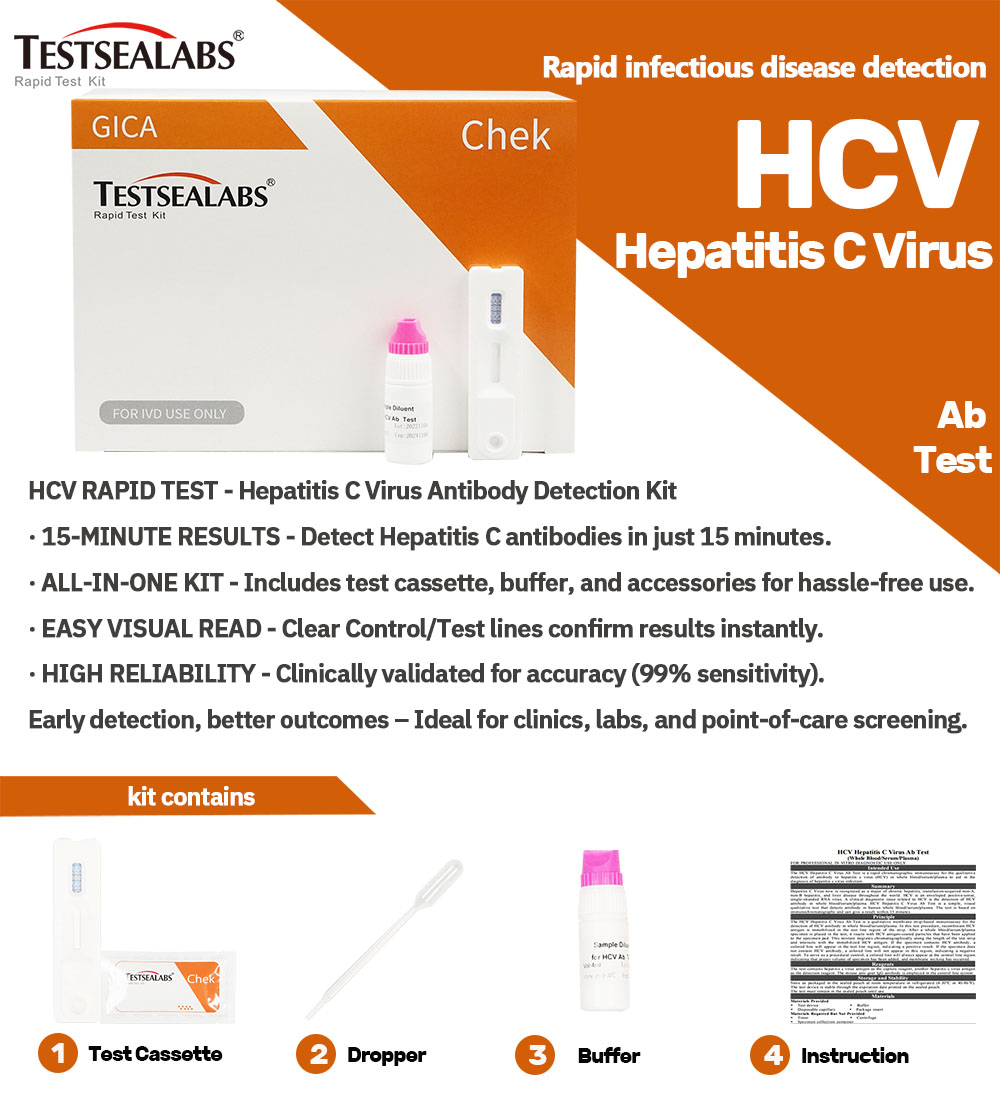
Detalia Producti:
- Alta Sensibilitate et Specificitate
Ad accurate detegendum designatumanticorpora anti-HCV, eventus certos cum minimo periculo falsorum positivorum vel falsorum negativorum praebens. - Resultata Celeria
Examen intra eventus praebetXV–XX minuta, decisiones opportunas de curatione aegrotorum et cura subsequenti facilitando. - Facile Usu
Examen facile administratur, nulla necessitate disciplinae vel instrumentorum specialis, ita ut aptum sit ad usum in variis condicionibus curationis valetudinis. - Genera Exemplorum Versatilia
Examen operatur cumsanguis totus, serum, velplasma, flexibilitatem in collectione exemplorum praebens. - Portatilis et ad usum in agro aptus
Designatio compacta et levis instrumenti probationis id aptum reddit adunitates valetudinis mobiles, programmata communicationis communis, etexpeditiones salutis publicae.
Modus Probationis:
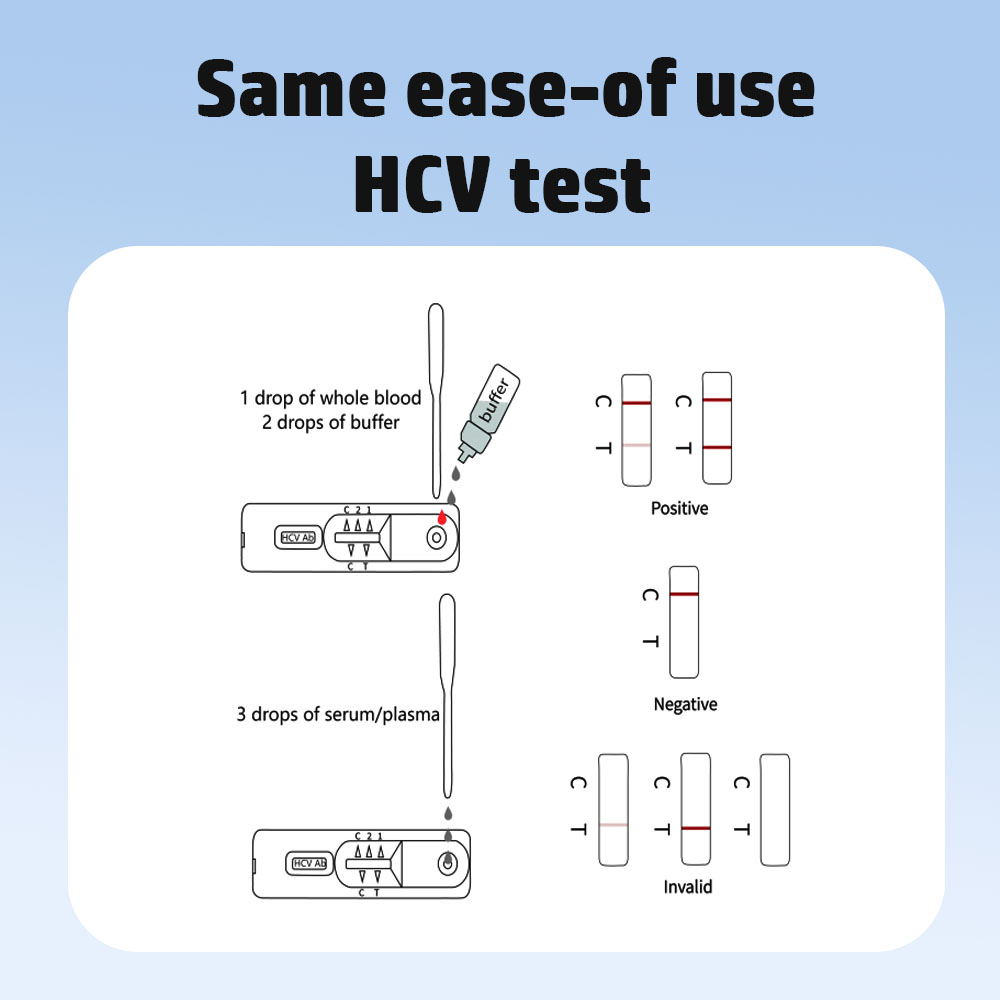
Instrumentum ad detegendum anticorpora Virus Hepatitis C (anti-HCV) in exemplo per immunochromatographiam (technologiam fluxus lateralis) operatur. Processus hos gradus comprehendit:
Additio Exempli
Parva quantitas sanguinis integri, seri, vel plasmatis in puteum exempli instrumenti probationis additur, una cum solutione tamponata.
Reactio Antigeni et Anticorporis
Cassetta probationis antigena HCV recombinantia continet, quae in linea probationis immobilizata sunt. Si anticorpora anti-HCV in exemplo adsunt, antigenis adhaerent et complexum antigeni-anticorporis formabunt.
Migratio Chromatographica
Complexus antigeni et anticorporis per membranam per actionem capillarem migrat. Si anticorpora anti-HCV adsunt, lineae probationis (lineae T) adhaerent, fasciam coloratam visibilem creantes. Reliqua reagentia ad lineam moderatricem (lineae C) migrabunt ut confirmetur probationem rite functionem esse.
Interpretatio Resultatorum
Duae lineae (linea T + linea C): Resultatum positivum, praesentiam anticorporum anti-HCV indicans.
Una linea (linea C tantum): Resultatum negativum, indicans nulla anticorpora anti-HCV detectabilia.
Nulla linea vel linea T tantum: Resultatum invalidum, probationem iteratam requirens.









