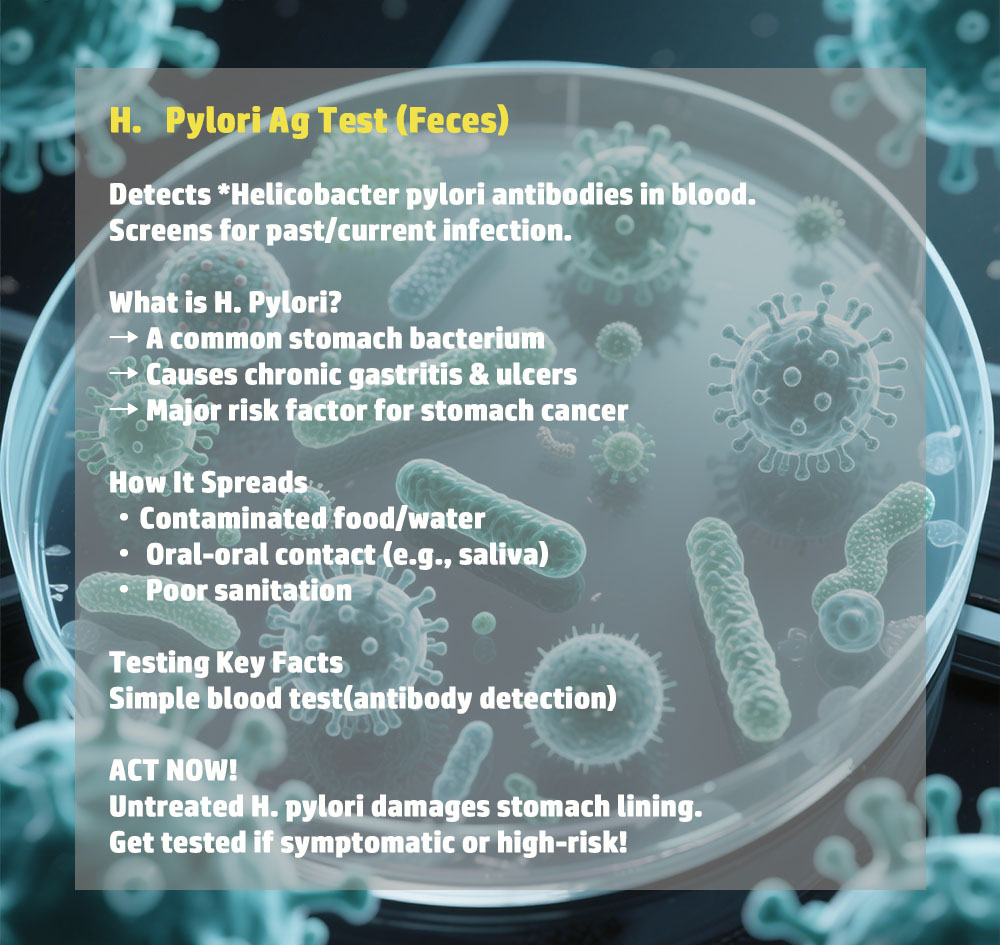
Instrumentum ad Morbum Testsealabs Examen H.Pylori Ag Celeris Examinis
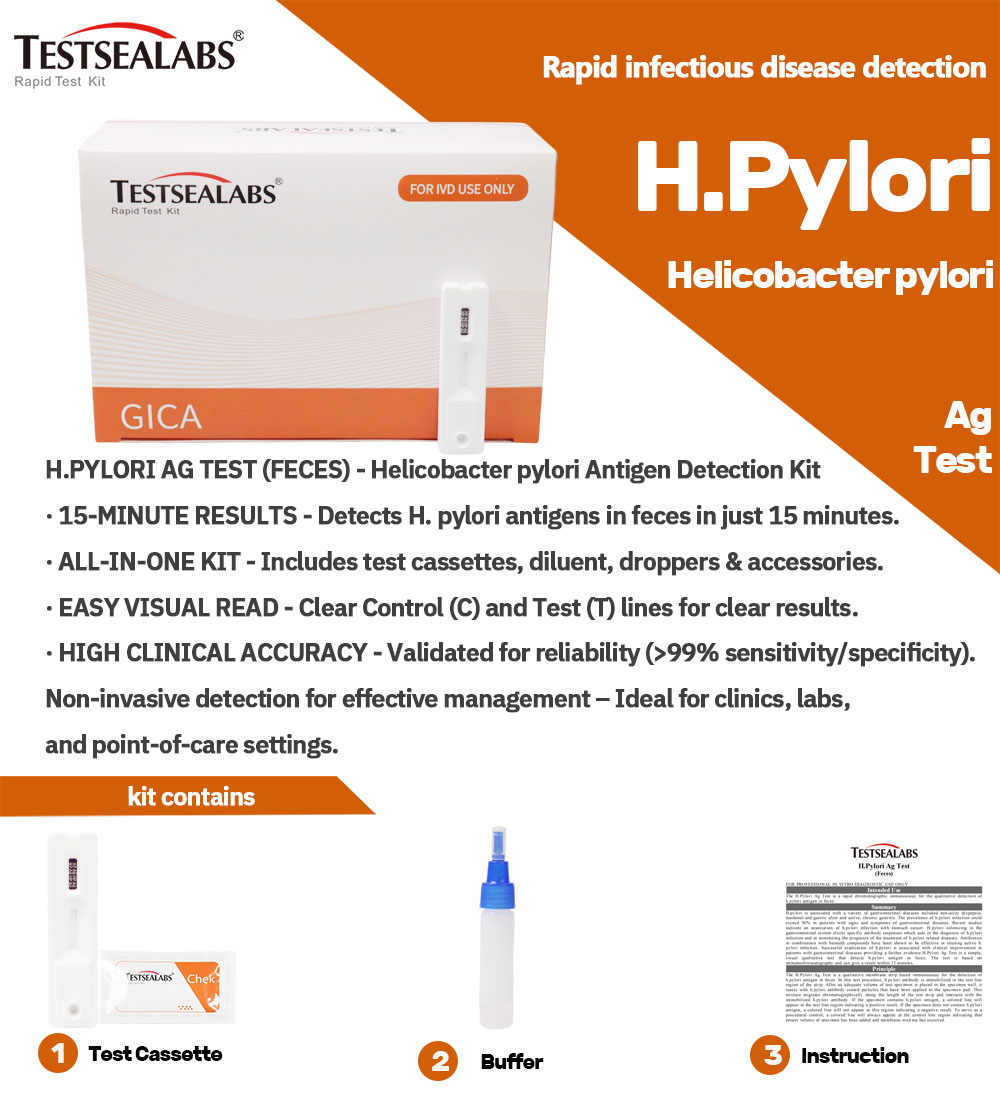
Detalia Producti:
- Alta Sensibilitate et Specificitate
Ad accurate detegendum designatumExamen H.Pylori Ag (Faecibus), eventus certos cum minimo periculo falsorum positivorum vel falsorum negativorum praebens. - Resultata Celeria
Examen intra eventus praebetQuindecim minuta, decisiones opportunas de curatione aegrotorum et cura subsequenti facilitando. - Facile Usu
Examen facile administratur, nulla necessitate disciplinae vel instrumentorum specialis, ita ut aptum sit ad usum in variis condicionibus curationis valetudinis. - Portatilis et ad usum in agro aptus
Designatio compacta et levis instrumenti probationis id aptum reddit adunitates valetudinis mobiles, programmata communicationis communis, etexpeditiones salutis publicae.
Modus Probationis:
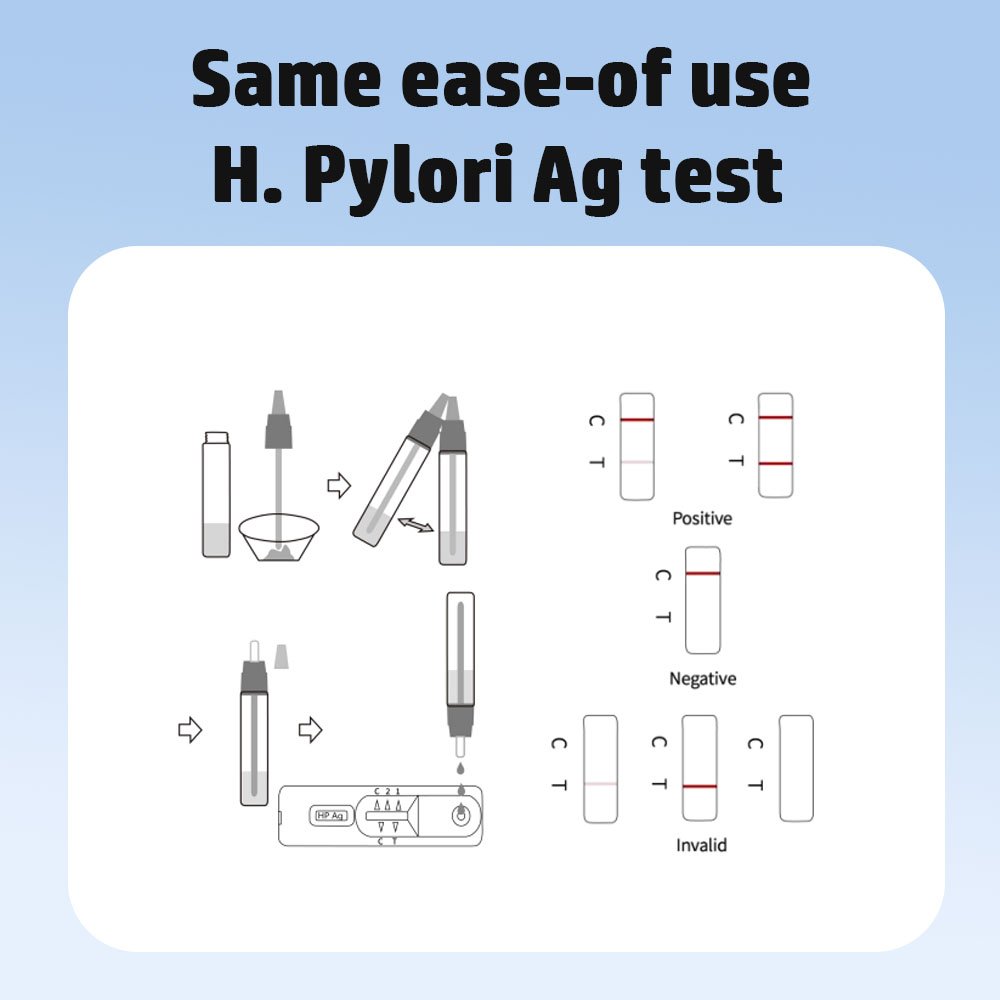
PositivumDuae lineae apparent. Una linea semper in regione lineae moderatoriae (C) apparere debet, et altera linea colorata conspicua in regione lineae probationis apparere debet.
NegativumUna linea colorata in regione moderatrice (C) apparet. Nulla linea colorata manifesta in regione lineae probationis apparet.
InvalidumLinea moderatoria non apparet. Volumen speciminis insufficiens vel rationes procedurales incorrectae sunt causae probabilissimae defectus lineae moderatoriae. Rationem recense et experimentum cum novo instrumento experimentali repete. Si problema perseverat, usum instrumenti experimentalis intermitte.statim et distributorem localem tuum contange.








