Influenza A&B Test Cassette
【INTENDED USE】
Testsealabs® Influenza A&B Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of influenza A and B antigens in nasal swab specimens. It is intended to aid in the rapid differential diagnosis of influenza A and B viral infections.
【Specification】
20pc/box (20 test devices+ 20 Extraction Tubes+1 Extraction Buffer+ 20 Sterilized Swabs+1 Product Insert)
1. Test Devices
2. Extraction Buffer
3. Extraction Tube
4. Sterilized Swab
5. Work Station
6. Package Insert

【SPECIMEN COLLECTION AND PREPARATION】
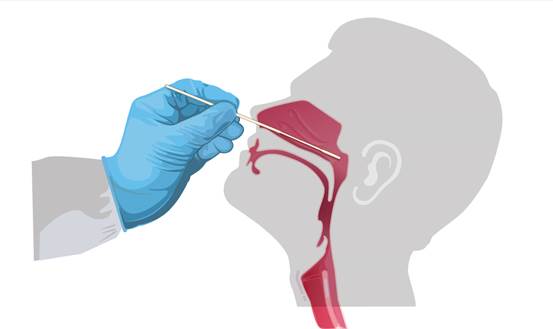
• Use the sterile swab supplied in the kit.
• Insert this swab into the nostril that presents the most secretion under
visual inspection.
• Using gentle rotation, push the swab until resistance is met at the level
of the turbinates (less than one inch in the nostril).
• Rotate the swab three times against the nasal wall.
It is recommended that swab specimens be processed as soon as
possible after collection. If swabs are not processed immediately they
should be placed into a dry, sterile, and tightly sealed plastic tube for
storage. Swabs can be stored dry at room temperature for up to 24
hours.
【DIRECTIONS FOR USE】
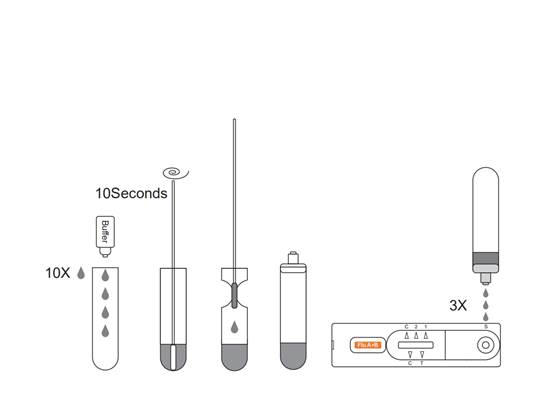
Allow the test, specimen, extraction buffer to equilibrate to roomtemperature (15-30°C) prior to testing.
1.Remove the test from the foil pouch and use it as soon as possible.
2.Place the Extraction Tube in the workstation. Hold the extraction reagent bottle upside down vertically. Squeeze the bottle and let the solution drop into the extraction tube freely without touching the edge of the tube. Add 10 drops of solution to the Extraction Tube.
3.Place the swab specimen in the Extraction Tube. Rotate the swab for approximately 10 seconds while pressing the head against the inside of the tube to release the antigen in the swab.
4.Remove the swab while squeezing the swab head against the inside of the Extraction Tube as you remove it to expel as much liquid as possible from the swab. Discard the swab in accordance with your biohazard waste disposal protocol.
5.Cover the tube with cap,then add 3 drops of the sample into the sample hole vertically.
6.Read the result after 15 minutes. If left unread for 20 minutes or more the results are invalid and a repeat test is recommended.
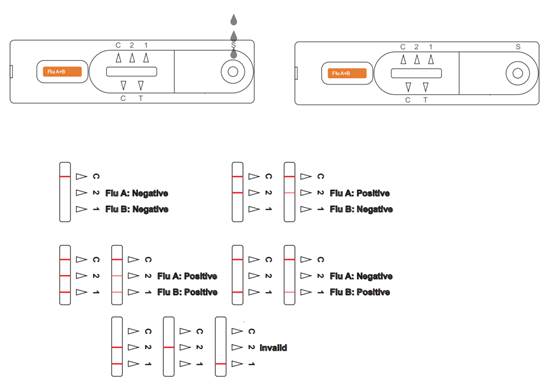
INTERPRETATION OF RESULTS
(Please refer to the illustration above)
POSITIVE Influenza A:* Two distinct colored lines appear. One line should be in the control line region (C) and another line should be in the Influenza A region (A). A positive result in the Influenza A region indicates that Influenza A antigen was detected in the sample.POSITIVE Influenza B:* Two distinct colored lines appear. One line should be in the control line region (C) and another line should be in the Influenza B region (B). A positive result in the Influenza B region indicates that Influenza B antigen was detected in the sample.
POSITIVE Influenza A and Influenza B: * Three distinct colored lines appear. One line should be in the control line region (C) and the other two lines should be in the Influenza A region (A) and Influenza B region (B). A positive result in the Influenza A region and Influenza B region indicates that Influenza A antigen and Influenza B antigen were detected in the sample.
*NOTE: The intensity of the color in the test line regions (A or B) will vary based on the amount of Flu A or B antigen present in the sample.So any shade of color in the test regions (A or B) should be considered positive.
NEGATIVE: One colored line appears in the control line region (C). No apparent colored line appears in the test line regions (A or B). A negative result indicates that Influenza A or B antigen is not found in the sample, or is there but below the detection limit of the test. The patient’s sample should be cultured to make sure that there is no Influenza A or B infection. If the symptoms do not agree with the results, get another sample for viral culture.
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.