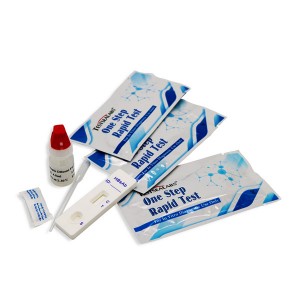
Nnwale Ọrịa Testsealabs HBsAb Ngwa ngwa ngwa ngwa
Nkọwa ngwa ngwa
| Aha ika: | testsea | Aha ngwaahịa: | HBsAb Hepatitis B Surface Antibody Test |
| Ebe amụrụ: | Zhejiang, China | Ụdị: | Ngwa nyocha ọrịa pathological |
| Asambodo: | ISO9001/13485 | Nhazi akụrụngwa | Klas II |
| izi ezi: | 99.6% | Ụdị: | Ọbara dum/Serum/Plasma |
| Usoro: | Kaseti/Nwapụta | Nkọwapụta: | 3.00mm / 4.00mm |
| MOQ: | 1000 PC | Ndụ nchekwa: | afọ 2 |

Ezubere iji
Otu nzọụkwụ HBsAb ule bụ ngwa ngwa chromatographic immunoassay maka qualitative nchọpụta nke ịba ọcha n'anya B elu Antibody (HBsAb) na Whole Blood / Serum / Plasma.

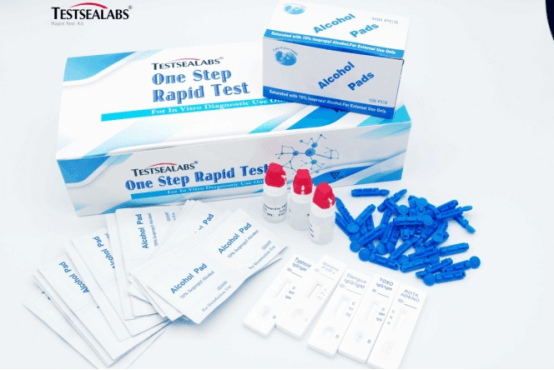
Nchịkọta
Ihe na-akpata ịba ọcha n'anya B bụ nje na-emetụta imeju. Ndị okenye nwere ịba ọcha n'anya B na-agbakekarị. Otú ọ dị, ọtụtụ ụmụ ọhụrụ bu ọrịa mgbe a mụrụ ha na-aghọ ndị na-ebu nje na-adịghị ala ala, ya bụ, ha na-ebu nje ahụ ruo ọtụtụ afọ ma nwee ike gbasaa ọrịa ahụ na ndị ọzọ.
Usoro ule
Kwe ka ule, ụdị, ihe nchekwa na/ma ọ bụ njikwa iru okpomọkụ 15-30℃ (59-86℉) tupu nnwale.
1. Weta obere akpa ahụ na okpomọkụ tupu imeghe ya. Wepu ngwaọrụ nnwale naobere akpa akara ma jiri ya ozugbo enwere ike.
2. Debe ngwaọrụ ule na elu dị ọcha na larịị.
3. Maka ụdị serum ma ọ bụ plasma: Jide dropper ahụ kwụ ọtọ ma bufee mmiri mmiri 3.ma ọ bụ plasma (ihe dị ka 100μl) gaa na ụdị nke ọma (S) nke ngwaọrụ nnwale, wee malitengụ oge. Lee ihe atụ n'okpuru.
4. Maka ụdị ọbara zuru oke: Jide ntụpọ ahụ kwụ ọtọ ma bufee 1 dobe nke dumọbara (ihe dị ka 35μl) na ụdị nke ọma (S) nke ngwaọrụ nnwale, wee tinye 2 tụlee nke ihe nchekwa (ihe dị ka 70μl) wee malite ngụ oge. Lee ihe atụ n'okpuru.
5. Chere ka ahịrị(s) nwere agba pụta. Gụọ nsonaazụ na nkeji iri na ise. Atụgharịla yapụta mgbe 20 nkeji.
Itinye ihe nlere zuru oke dị mkpa maka nsonaazụ ule dị mma. Ọ bụrụ na mbugharị (wettingA naghị ahụ nke akpụkpọ ahụ) na windo ule mgbe otu nkeji gachara, tinye otu dobe nchekwa ọzọ(maka ọbara zuru oke) ma ọ bụ ụdị (maka serum ma ọ bụ plasma) na ihe nlele ahụ nke ọma.
Nkọwa nke nsonaazụ
Nke dị mma:Ahịrị abụọ pụtara. Otu ahịrị kwesịrị ịpụta mgbe niile na mpaghara akara akara (C), naọzọ otu doro anya na-acha ahịrị kwesịrị ịpụta na mpaghara ahịrị ule.
adịghị mma:Otu ahịrị na-acha na-egosi na mpaghara njikwa(C).Ọnweghị ahịrị agba pụtara ìhè pụtarampaghara akara ule.
Na ezighi ezi:Ahịrị njikwa agaghị apụta. Oke ihe atụ ezughi oke ma ọ bụ usoro ezighi eziusoro bụ ihe yikarịrị kpatara ọdịda akara akara.
★ Nyochaa usoro ma kwugharịaule na ngwaọrụ ule ọhụrụ. Ọ bụrụ na nsogbu ahụ dịgidere, kwụsị iji ngwa nnwale ozugbo wee kpọtụrụ onye nkesa mpaghara gị.
Ozi ngosi






Nkọwapụta Ụlọ ọrụ
Anyị, Hangzhou Testsea Biotechnology Co., Ltd bụ ụlọ ọrụ ọkachamara biotechnology na-eto ngwa ngwa ọkachamara na nyocha, mmepe, nrụpụta na nkesa nke ngwa ule in-vitro diagnostics (IVD) dị elu na ngwa ahụike.
Ụlọ ọrụ anyị bụ GMP, ISO9001, na ISO13458 kwadoro ma anyị nwere nkwenye CE FDA. Ugbu a, anyị na-atụ anya isonyere ọtụtụ ụlọ ọrụ esenidụt maka mmepe otu.
Anyị na-emepụta ule ọmụmụ, ule ọrịa na-efe efe, ule iji ọgwụ ọjọọ eme ihe, ule akara obi, ule akara tumor, ule nri na nchekwa na ule ọrịa anụmanụ, na mgbakwunye, akara TESTSEALABS bụ nke ama ama n'ahịa ụlọ na mba ofesi. Ogo kacha mma na ọnụ ahịa dị mma na-enyere anyị aka iweghara 50% nke oke ụlọ.
Usoro ngwaahịa

1. Kwadebe

2.Mkpuchi

3.Cross akpụkpọ ahụ

4.Ibe warara

5.Mgbakọ

6. Kpochie obere akpa

7.Achie akpa ahụ

8.Pack igbe

9. Ihe mkpuchi