Testsealabs HCG Pregnancy Test Cassette
Parameter table
| Model Number | HCG |
| Name | HCG Pregnancy Test Cassette |
| Features | High sensitivity, Simple, Easy and Accurate |
| Specimen | Urine |
| Sensitivity | 10-25mIU/ml |
| Accuracy | > 99% |
| Storage | 2'C-30'C |
| Shipping | By sea/By air/TNT/Fedx/DHL |
| Instrument classification | Class II |
| Certificate | CE/ ISO13485 |
| Shelf life | two years |
| Type | Pathological Analysis Equipments |
Principle of HCG Cassette Rapid Test Device
Because the amount of a hormone called human chorionic gonadotropin (hCG) in your body increases rapidly during the first two weeks of pregnancy, the test cassette will detect the presence of this hormone in your urine as early as the first day of a missed period. The test cassette can accurately detect pregnancy when the level of hCG is between 25mIU/ml to 500,000mIU/ml.
The test reagent is exposed to urine, allowing urine to migrate through the absorbent test cassette. The labeled antibody-dye conjugate binds to the hCG in the specimen forming an antibody-antigen complex. This complex binds to the anti-hCG antibody in the test region (T) and produces a red line when hCG concentration is equal to or greater than 25mIU/ml. In the absence of hCG, there is no line in the test region (T). The reaction mixture continues flowing through the absorbent device past the test region (T) and control region (C). Unbound conjugate binds to the reagents in the control region (C), producing a red line, demonstrating that the test cassette is functioning correctly.

TEST PROCEDURE
Read the entire procedure carefully before performing any tests.
Allow test strip and urine specimen to equilibrate to room temperature (20-30℃ or 68-86℉) prior to testing.
1.Remove the test strip from the sealed pouch.
2.Holding the strip vertically, carefully dip it into the specimen with the arrow end pointing towards the urine.
NOTE: Do not immerse the strip past the Max Line.
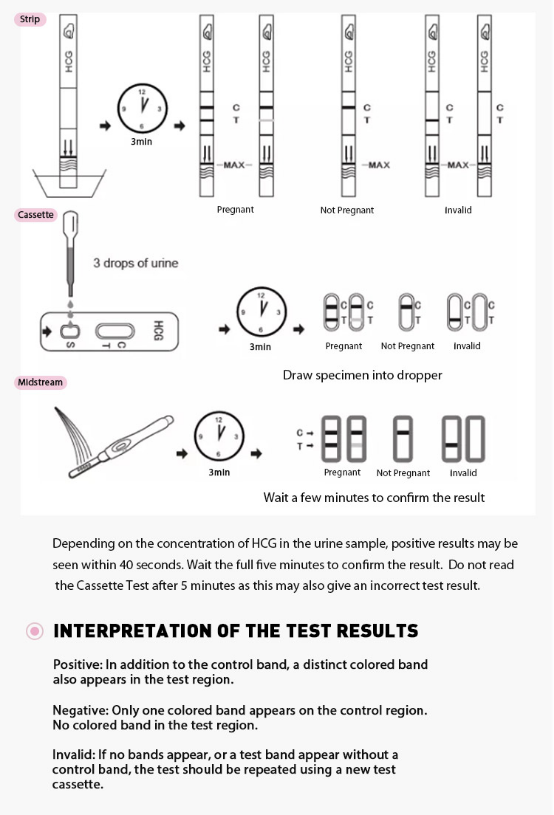
3.Wait for colored lines to appear. Interpret the test results at 3-5 minutes.
NOTE: Do not read results after 10 minutes.
CONTENTS, STORAGE AND STABILITY
The test strip consists of colloidal gold-monoclonal antibody against LH coated on polyester membrane, and monoclonal antibody against LH and goat-anti-mouse IgG coated on cellulose nitrate membrane.
Each pouch contains one test strip and one desiccant.
INTERPRETATION OF RESULTS
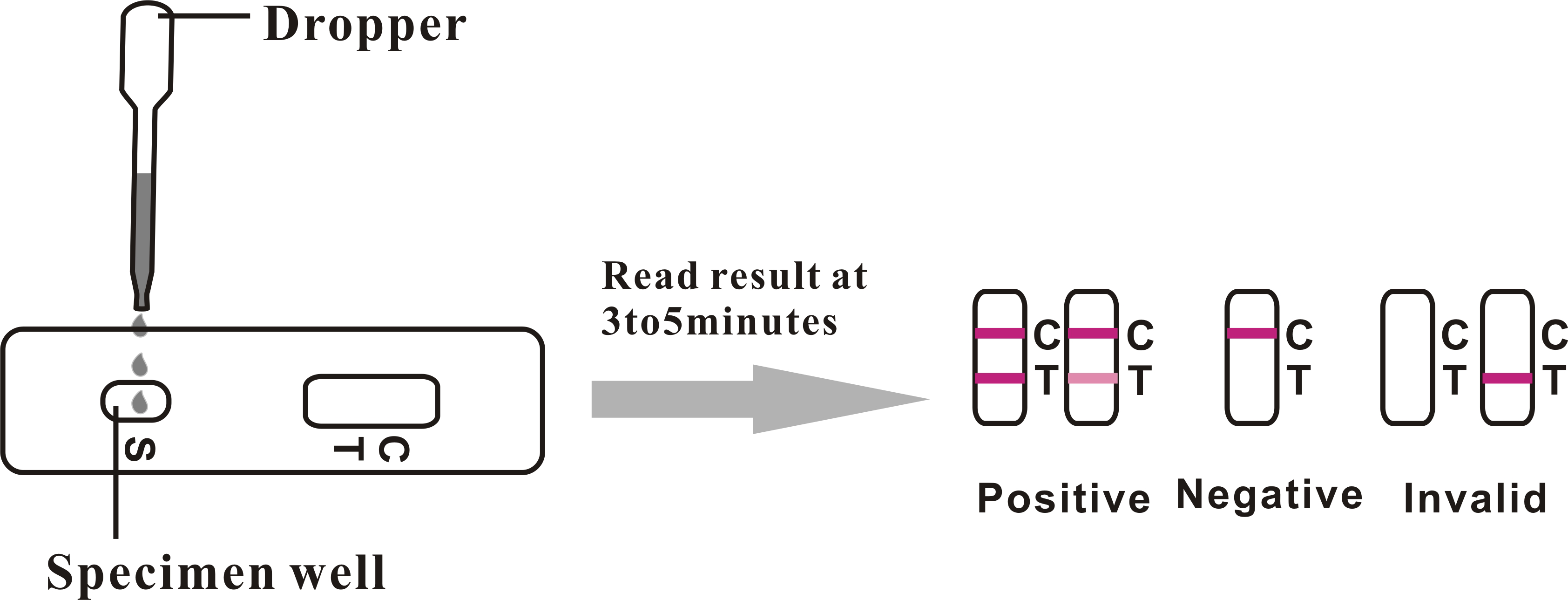
Positive (+)
Two distinct red lines will appear, one in the test region (T) and another in the control region (C). You can assume that you are pregnant.
Negative (-)
Only one red line appears in the control region (C). No apparent line in the test region (T). You can assume that you are not pregnant.
Invalid
The result is invalid if no red line appears in the control region (C), even if a line appears in the test region (T). In any event, repeat the test. If the problem persists, discontinue using the lot immediately and contact your local distributor.
NOTE: Clear background in the Result Window can be seen as a basis for effective testing. If the test line is weak, it is recommended that the test be repeated with the first morning specimen obtained 48-72 hours later. No matter how the test results, it is recommended to consult your physician.
Performance Characteristics
Exhibition Information






Company Profile
We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
Product Process

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement



