FSH Follicle Stimulating HormoneTest Kit
Parameter table
| Model Number | HFSH |
| Name | FSH Menopause Urine Test Kit |
| Features | High sensitivity, Simple, Easy and Accurate |
| Specimen | Urine |
| Specification | 3.0mm 4.0mm 5.5mm 6.0mm |
| Accuracy | > 99% |
| Storage | 2'C-30'C |
| Shipping | By sea/By air/TNT/Fedx/DHL |
| Instrument classification | Class II |
| Certificate | CE/ ISO13485 |
| Shelf life | two years |
| Type | Pathological Analysis Equipments |

Principle of FSH Rapid Test Device
1.SAMPLE COLLECTION AND HANDLING
To perform this test, collect a urine sample in a clean and dry container. Fresh urine does not require any special handing or pretreatment. Testing should be performed as soon as possible after the specimen collection, preferably during the same day. The specimen may be refrigerated at 2-8℃ for 3 days or frozen at -20℃ for a longer period of time. Specimens that have been refrigerated must be equilibrated to room temperature prior to testing. Specimens previously frozen must be thawed, equilibrated to room temperature, and mixed thoroughly prior to testing.
2.TO CARRY OUT THE TES
3.DIRECTIONS FOR USE
1) The test is formulated for use with fresh urine specimens. Wear gloves and use the urine cup to collect urine.
2)Remove the test cassette from its foil pouch.
3) Draw urine sample into dropper, and dispense it into the sample well on the cassette (2-3 drops, approximately 100μl). Be careful not to overfill the absorbent pad.
4)Read results in 5 minutes.
5)Discard the test device after a single use.
Note: Please Wait the full 5 minutes to confirm the result. Do not read the one step FSH Test after 5 minutes as this may give an incorrect test result. This is a single use test. Please safely dispose of the strip, regard this as infectious material and dispose of the test appropriately after use.
CONTENTS, STORAGE AND STABILITY
Each box contains: 3 foil pouches and Operating Instructions.
Each pouch contains: 1Step Follicle Stimulating Hormone(FSH) Test Strip and 1 Desiccant.
The test kit can be stored at room temperature (35.6F-86F; 2℃-30℃) in the sealed pouch to the date of expiration. The test kits should be kept away from direct sunlight, moisture and heat. Do not freeze.
MATERIALS REQUIRED BUT NOT PROVIDED
Specimen collection container and timer
For Strip Specimen
1.Remove the FSH test strip out from the sealed pouch.
2. Immerse the test strip with arrow downside into the urine for about 5 seconds and lay the strip flat on a clean, dry, non-absorbent surface .Do not exceed the marker line.
3.Wait for red lines to appear. Depending on the concentration of FSH in the test specimen, positive results may be observed in as short as 60 seconds. However, to confirm negative results, the complete reaction time (5 minutes) is required. Do not read results after 10 minutes.
For cassette specimens:
1.Remove the test cassette from the sealed pouch.
2.Hold the dropper vertically and transfer 3 full drops of urine to the specimen well of the test cassette, and then begin timing.
3.Wait for colored lines to appear. Interpret the test results at 3-5 minutes.
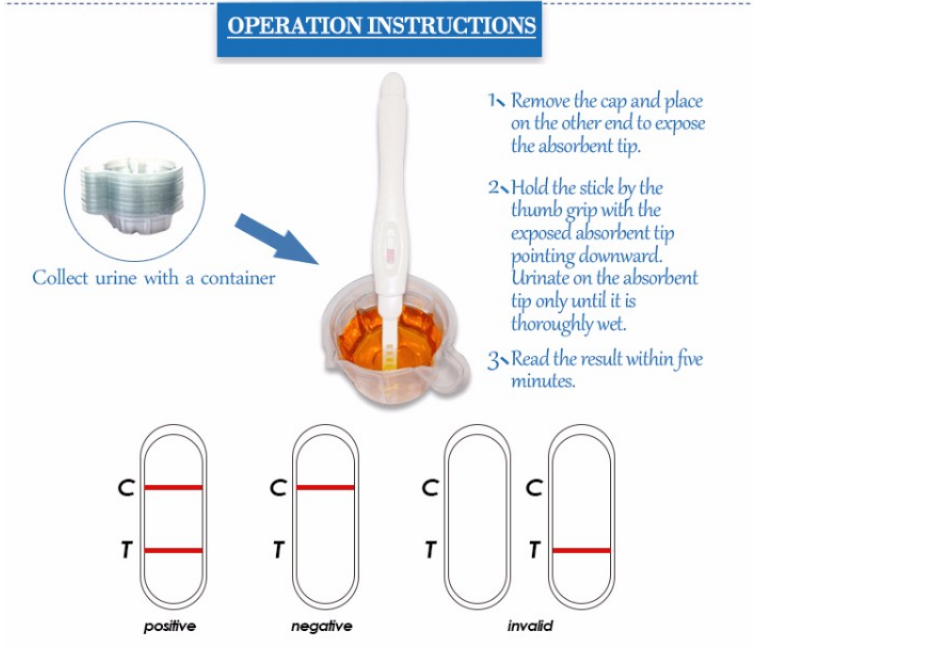
INTERPRETATION OF RESULTS
Positive (+)
In addition to one purple band in the control region (C), a purple band will appear in the test region (T).
Negative (-)
No apparent band in the test region (T), Only one purple band appears in the control
region (C).
Invalid
No visibie bang at all or no colored band appears on the control region (C) .Repeat test with a new test kit.
Exhibition Information






Company Profile
We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
Product Process

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement




