Flu A/B + COVID-19 Antigen Combo Test
【INTENDED USE】
Testsealabs® The test is intended for use in the simultaneous rapid in vitro detection and differentiation of influenza A virus, influenza B virus, and COVID-19 virus nucleocapsid protein antigen , but does not differentiate, between SARS-CoV and COVID-19 viruses and is not intended to detect influenza C antigens. Performance characteristics may vary against other emerging influenza viruses. Influenza A, influenza B, and COVID-19 viral antigens are generally detectable in upper respiratory specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative COVID-19 results, from patients with symptom onset beyond five days, should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed. Negative results do not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19. Negative results do not preclude influenza virus infections and should not be used as the sole basis for treatment or other patient management decisions.
【Specification】
250pc/box (25 test devices+ 25 Extraction Tubes+25 Extraction Buffer+ 25Sterilized Swabs+1 Product Insert)
1. Test Devices
2. Extraction Buffer
3. Extraction Tube
4. Sterilized Swab
5. Work Station
6. Package Insert

【SPECIMEN COLLECTION AND PREPARATION】
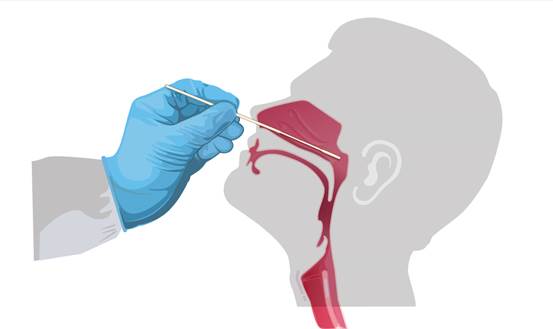
Swab Specimen collection 1. Only the swab provided in the kit is to be used for nasopharyngeal swab collection. To collect a nasopharyngeal wab sample, carefully insert the swab into the nostril exhibiting the most visible drainage, or the nostril that is most congested if drainage is not visible. Using gentle rotation, push the swab until resistance is met at the level of the turbinates (less than one inch into the nostril). Rotate the swab 5 times or more against the nasal wall then slowly remove from the nostril. Using the same swab, repeat sample collection in the other nostril. 2. Flu A/B + COVID-19 Antigen Combo Test Cassette can be applied to nasopharyngeal swab. 3. Do not return the nasopharyngeal swab to the original paper packaging. 4. For best performance, direct nasopharyngeal swabs should be tested as soon as possible after collection. If immediate testing is not possible, and to maintain best performance and avoid possible contamination, it is highly recommended the nasopharyngeal swab is placed in a clean, unused plastic tube labeled with patient information, preserving sample integrity, and capped tightly at room temperature (15-30°C) for up to 1 hour prior to testing. Ensure the swab fits securely within the tube and the cap is tightly closed. If greater than 1 hour delay occurs, dispose of sample. A new sample must be collected for testing. 5. If specimens are to be transported, they should be packed in compliance with local regulations covering the transportation of etiologicalagents
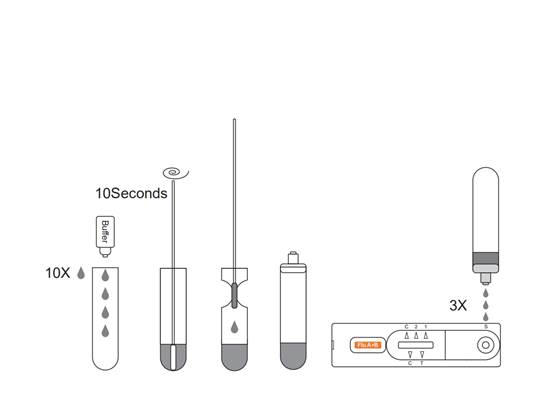
【DIRECTIONS FOR USE】
Allow the test, specimen, buffer and/or controls to reach room temperature 15-30℃ (59-86℉) prior to testing. 1. Place the Extraction Tube in the workstation. Hold the extraction reagent bottle upside down vertically. Squeeze the bottle and let the solution drop into the extraction tube freely without touching the edge of the tube. Add 10 drops of solution to the Extraction Tube. 2.Place the swab specimen in the Extraction Tube. Rotate the swab for approximately 10 seconds while pressing the head against the inside of the tube to release the antigen in the swab. 3.Remove the swab while squeezing the swab head against the inside of the Extraction Tube as you remove it to expel as much liquid as possible from the swab. Discard the swab in accordance with your biohazard waste disposal protocol. 4.Cover the tube with cap,then add 3 drops of the sample into the left sample hole vertically and add another 3 drops of the sample into the right sample hole vertically. 5.Read the result after 15 minutes. If left unread for 20 minutes or more the results are invalid and a repeat test is recommended.
INTERPRETATION OF RESULTS
(Please refer to the illustration above)
POSITIVE Influenza A:* Two distinct colored lines appear. One line should be in the control line region (C) and another line should be in the Influenza A region (A). A positive result in the Influenza A region indicates that Influenza A antigen was detected in the sample.
POSITIVE Influenza B:* Two distinct colored lines appear. One line should be in the control line region (C) and another line should be in the Influenza B region (B). A positive result in the Influenza B region indicates that Influenza B antigen was detected in the sample.
POSITIVE Influenza A and Influenza B: * Three distinct colored lines appear. One line should be in the control line region (C) and the other two lines should be in the Influenza A region (A) and Influenza B region (B). A positive result in the Influenza A region and Influenza Bregion indicates that Influenza A antigen and Influenza B antigen were detected in the sample.
*NOTE: The intensity of the color in the test line regions (A or B) will vary based on the amount of Flu A or B antigen present in the sample. So any shade of color in the test regions (A or B) should be considered positive.
NEGATIVE: One colored line appears in the control line region (C).
No apparent colored line appears in the test line regions (A or B). Anegative result indicates that Influenza A or B antigen is not found in the sample, or is there but below the detection limit of the test. The patient’s sample should be cultured to make sure that there is no Influenza A or B infection. If the symptoms do not agree with the results, get another sample for viral culture.
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
【INTERPRETATION OF RESULTS】 Interpretation of Flu A/B results(On the left) Influenza A Virus POSITIVE:* Two colored lines appear. One colored line should always appear in the control line region (C) and another line should be in the Flu A line region (2). Influenza B Virus POSITIVE:* Two colored lines appear. One colored line should always appear in the control line region (C) and another line should be in the Flu B line region(1). Influenza A Virus andInfluenza B Virus POSITIVE:* Three colored lines appear. One colored line should always appear in the control line region (C) and two test lines should be in the Flu A line region (2) and Flu B line region(1) *NOTE: The intensity of the color in the test line regions may vary depending on the
concentration of influenza A virus and influenza B virus present in the specimen. Therefore, any shade of color in the test line region should be considered positive. Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line regions. Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
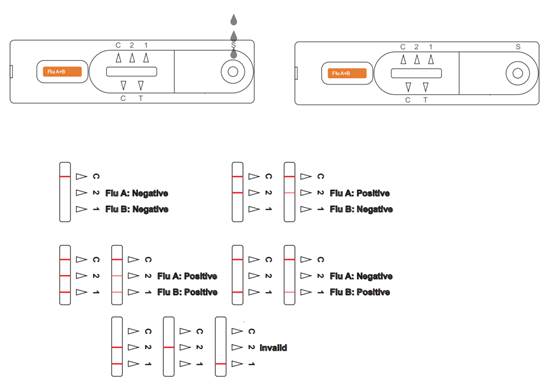
Interpretation of COVID-19 antigen results(On the right) Positive: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region(T). *NOTE: The intensity of the color in the test line regions may vary depending on the concentration of COVID-19 antigen present in the specimen. Therefore, any shade of color in the test line region should be considered positive. Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region(T). Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.


