Dengue IgM/IgG/NS1 Antigen Test Dengue Combo Test
Dengue is transmitted by the bite of an Aedes mosquito infected with any one of the four dengue viruses. It occurs in tropical and sub-tropical areas of the world. Symptoms appear 3 — 14 days after the infective bite. Dengue fever is a febrile illness that affects infants, young children and adults. Dengue haemorrhagic fever (fever, abdominal pain, vomiting, bleeding) is a potentially lethal complication, affecting mainly children. Early clinical
diagnosis and careful clinical management by experienced physicians and nurses increase survival of patients. One step Dengue NS1 Test is a simple, visual qualitative test that detects dengue virus antibodies in human Whole Blood/serum/plasma. The test is based on immunochromatography and can give a result within 15 minutes.
INBasic Info.
|
Model No |
101011 |
Storage Temperature |
2-30 Degree |
|
Shelf Life |
24M |
Delivery Time |
Within 7 working days |
|
Diagnostic target |
Dengue NS1 Virus |
Payment |
T/T Western Union Paypal |
|
Transport Package |
Carton |
Packing Unit |
1 Test device x 10/kit |
| Origin | China | HS Code | 38220010000 |
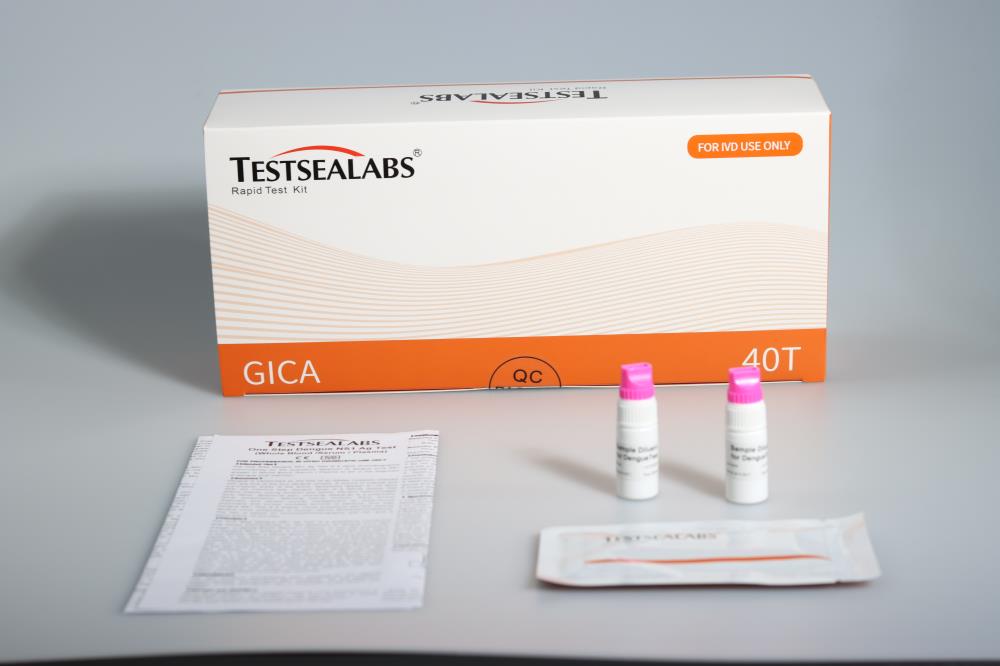
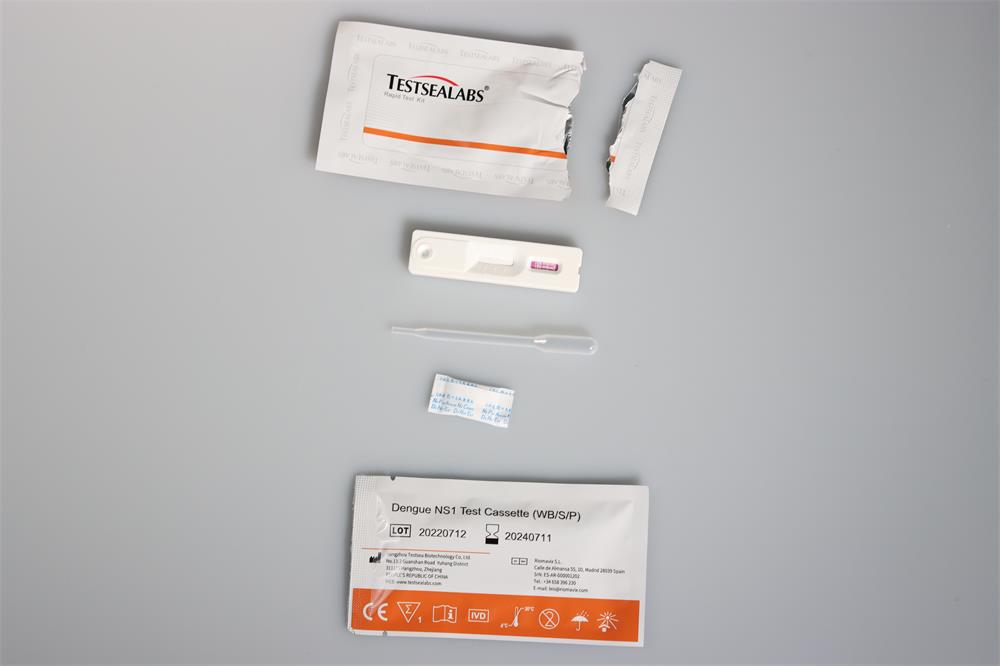
Materials Provided
1.Testsealabs test device individually foil-pouched with a desiccant
2.Assay solution in dropping bottle
3.Instruction manual for use
Feature
1. Easy opertaion
2. Fast read Result
3. High Sensitivity and accuracy
4. Reasonable price and high quality

Specimens Collection and Preparation
1.The One Step Dengue NS1 Ag Test can be performed used on Whole Blood /Serum / Plasma.
2.To collect whole blood, serum or plasma specimens following regular clinical laboratory procedures.
3.Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear non-hemolyzed specimens.
4.Testing should be performed immediately after specimen collection. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8 ℃ for up to 3 days. For long term storage, specimens should be kept below -20℃. Whole blood should be stored at 2-8 ℃ if the test is to be run within 2 days of collection. Do not freeze whole blood specimens.
5.Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
Test Procedure
Allow the test, specimen, buffer and/or controls to reach room temperature 15-30℃ (59-86℉) prior to testing.
1.Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible.
2.Place the test device on a clean and level surface.
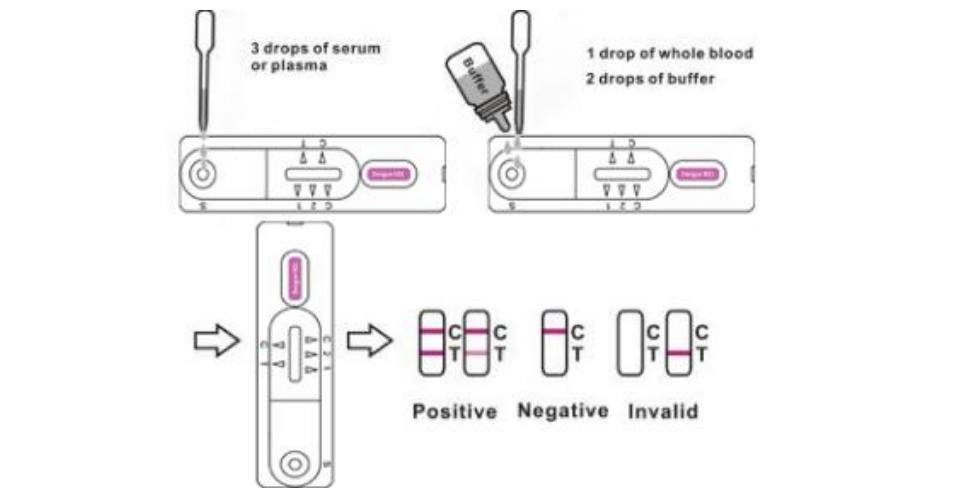
3.For serum or plasma specimen: Hold the dropper vertically and transfer 3 drops of serum or plasma (approximately 100μl) to the specimen well(S) of the test device, then start the timer. See illustration below.
4.For whole blood specimens: Hold the dropper vertically and transfer 1 drop of whole blood(approximately 35 μ l) to the specimen well(S) of the test device, then add 2 drops of buffer (approximately 70μl) and start the timer. See illustration below. Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.
Notes:
Applying sufficient amount of specimen is essential for a valid test result. If migration (the wetting of membrane) is not observed in the test window after one minute, add one more drop of buffer (for whole blood) or specimen (for serum or plasma) to the specimen well.
Interpretation of Result
Positive: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line
should appear in the test line region.
Negative: One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Company Profile

Other infectious disease test we supply
| Infectious Disease Rapid Test Kit |
|
||||||
|
Product name |
Catalog No. |
Specimen |
Format |
Specification |
|
Certificate |
|
|
Influenza Ag A Test |
101004 |
Nasal/Nasopharyngeal Swab |
Cassette |
25T |
|
CE ISO |
|
|
Influenza Ag B Test |
101005 |
Nasal/Nasopharyngeal Swab |
Cassette |
25T |
|
CE ISO |
|
|
HCV Hepatitis C Virus Ab Test |
101006 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
HIV 1/2 Test |
101007 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
HIV 1/2 Tri-line Test |
101008 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
HIV 1/2/O Antibody Test |
101009 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
Dengue IgG/IgM Test |
101010 |
WB/S/P |
Cassette |
40T |
|
CE ISO |
|
|
Dengue NS1 Antigen Test |
101011 |
WB/S/P |
Cassette |
40T |
|
CE ISO |
|
|
Dengue IgG/IgM/NS1 Antigen Test |
101012 |
WB/S/P |
Dipcard |
40T |
|
CE ISO |
|
|
H.Pylori Ab Test |
101013 |
WB/S/P |
Cassette |
40T |
|
CE ISO |
|
|
H.Pylori Ag Test |
101014 |
Feces |
Cassette |
25T |
|
CE ISO |
|
|
Syphilis(Anti-treponemia Pallidum) Test |
101015 |
WB/S/P |
Strip/Cassette |
40T |
|
CE ISO |
|
|
Typhoid IgG/IgM Test |
101016 |
WB/S/P |
Strip/Cassette |
40T |
|
CE ISO |
|
|
Toxo IgG/IgM Test |
101017 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
TB Tuberculosis Test |
101018 |
WB/S/P |
Strip/Cassette |
40T |
|
CE ISO |
|
|
HBsAg Hepatitis B surface Antigen Test |
101019 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
HBsAb Hepatitis B surface Antibody Test |
101020 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
HBsAg Hepatitis B virus e Antigen Test |
101021 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
HBsAg Hepatitis B virus e Antibody Test |
101022 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
HBsAg Hepatitis B virus core Antibody Test |
101023 |
WB/S/P |
Cassette |
40T |
|
ISO |
|
|
Rotavirus Test |
101024 |
Feces |
Cassette |
25T |
|
CE ISO |
|
|
Adenovirus Test |
101025 |
Feces |
Cassette |
25T |
|
CE ISO |
|
|
Norovirus Antigen Test |
101026 |
Feces |
Cassette |
25T |
|
CE ISO |
|
|
HAV Hepatitis A virus IgM Test |
101027 |
WB/S/P |
Cassette |
40T |
|
CE ISO |
|
|
HAV Hepatitis A virus IgG/IgM Test |
101028 |
WB/S/P |
Cassette |
40T |
|
CE ISO |
|
|
Malaria Ag p.f/p.v Tri-line Test |
101029 |
WB |
Cassette |
40T |
|
CE ISO |
|
|
Malaria Ag p.f/pan Tri-line Test |
101030 |
WB |
Cassette |
40T |
|
CE ISO |
|
|
Malaria Ag p.v Test |
101031 |
WB |
Cassette |
40T |
|
CE ISO |
|
|
Malaria Ag p.f Test |
101032 |
WB |
Cassette |
40T |
|
CE ISO |
|
|
Malaria Ag pan Test |
101033 |
WB |
Cassette |
40T |
|
CE ISO |
|
|
Leishmania IgG/IgM Test |
101034 |
Serum/Plasma |
Cassette |
40T |
|
CE ISO |
|
|
Leptospira IgG/IgM Test |
101035 |
Serum/Plasma |
Cassette |
40T |
|
CE ISO |
|
|
Brucellosis(Brucella)IgG/IgM Test |
101036 |
WB/S/P |
Strip/Cassette |
40T |
|
CE ISO |
|
|
Chikungunya IgM Test |
101037 |
WB/S/P |
Strip/Cassette |
40T |
|
CE ISO |
|
|
Chlamydia trachomatis Ag Test |
101038 |
Endocervical Swab/Urethral swab |
Strip/Cassette |
25T |
|
ISO |
|
|
Neisseria Gonorrhoeae Ag Test |
101039 |
Endocervical Swab/Urethral swab |
Strip/Cassette |
25T |
|
CE ISO |
|
|
Chlamydia Pneumoniae Ab IgG/IgM Test |
101040 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Chlamydia Pneumoniae Ab IgM Test |
101041 |
WB/S/P |
Strip/Cassette |
40T |
|
CE ISO |
|
|
Mycoplasma Pneumoniae Ab IgG/IgM Test |
101042 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Mycoplasma Pneumoniae Ab IgM Test |
101043 |
WB/S/P |
Strip/Cassette |
40T |
|
CE ISO |
|
|
Rubella virus antibody IgG/IgM test |
101044 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Cytomegalovirus antibody IgG/IgM test |
101045 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Herpes simplex virus Ⅰ antibody IgG/IgM test |
101046 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Herpes simplex virus ⅠI antibody IgG/IgM test |
101047 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Zika virus antibody IgG/IgM test |
101048 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Hepatitis E virus antibody IgM test |
101049 |
WB/S/P |
Strip/Cassette |
40T |
|
ISO |
|
|
Influenza Ag A+B Test |
101050 |
Nasal/Nasopharyngeal Swab |
Cassette |
25T |
|
CE ISO |
|
|
HCV/HIV/SYP Multi Combo Test |
101051 |
WB/S/P |
Dipcard |
40T |
|
ISO |
|
|
MCT HBsAg/HCV/HIV Multi Combo Test |
101052 |
WB/S/P |
Dipcard |
40T |
|
ISO |
|
|
HBsAg/HCV/HIV/SYP Multi Combo Test |
101053 |
WB/S/P |
Dipcard |
40T |
|
ISO |
|
|
Monkey Pox Antigen Test |
101054 |
oropharyngeal swabs |
Cassette |
25T |
|
CE ISO |
|
|
Rotavirus/Adenovirus Antigen Combo Test |
101055 |
Feces |
Cassette |
25T |
|
CE ISO |
|








