CEA Carcinoembryonic Antigen Test Kit
Parameter table
| Model Number | TSIN101 |
| Name | AFP Alpha-Fetoprotein Test Kit |
| Features | High sensitivity, Simple, Easy and Accurate |
| Specimen | WB/S/P |
| Specification | 3.0mm 4.0mm |
| Accuracy | 99.6% |
| Storage | 2'C-30'C |
| Shipping | By sea/By air/TNT/Fedx/DHL |
| Instrument classification | Class II |
| Certificate | CE ISO FSC |
| Shelf life | two years |
| Type | Pathological Analysis Equipments |
Principle of FOB Rapid Test Device
The CEA Rapid Test Device (Whole Blood/Serum/Plasma) has been designed to detect human carcinoembryonic antigen (CEA) through visual interpretation of color development in the internal strip. The membrane was immobilized with anti-CEA capture antibodies on the test region. During the test, the specimen is allowed to react with colored anti-CEA monoclonal antibodies colloidal gold conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by a capillary action, and interact with reagents on the membrane. If there were enough CEA in specimens, a colored band will form at the test region of the membrane. Presence of this colored band indicates a positive result, while its absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.

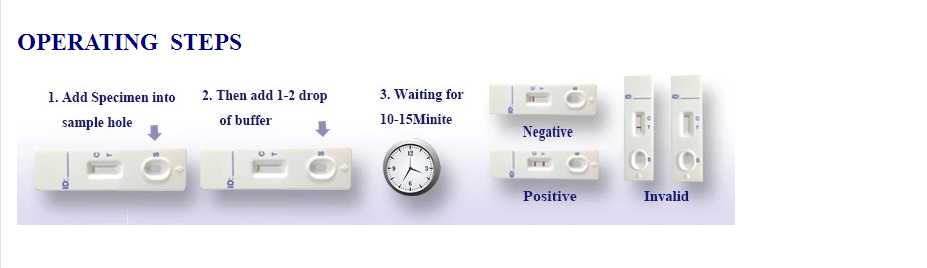
1.Do not open foil pouch until ready to begin testing. Refrigerated test devices should be allowed to come to room temperature (15°- 28°C) before opening the pouch.
2.Remove the device from the protective pouch and label the device with specimen identification.
3. Add 50 ul of fresh blood to the Sample Well (for Card) or Sample Pad (for Dipstick),Then add 2 drops (50 ul) of test running buffer into the sample well or sample pad.
4. Read the result within10- 15 minutes. Do not read results after15 minutes. Observe
the colored band developed over the control region indicating the assay is complete.
Test Procedure
CONTENT OF THE KIT
1.Individually packed test devices
Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding regions.
2.Disposable pipettes
For adding specimens use.
3.Buffer
Phosphate buffered saline and preservative.
4.Package insert
For operation instruction.
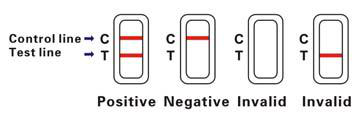
INTERPRETATION OF RESULTS
Positive (+)
Two pink bands appear on test region. This indicates that the specimen contains CEA
Negative (-)
Only one pink band appears on test region. This indicates that there is no CEA in the whole blood.
Invalid
If without colored band appears on test region, this is an indication of a possible error in performing the test. The test should be repeated using a new device.
Exhibition Information






Company Profile
We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
Product Process

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement