Avian Influenza Virus H9 Antigen Test
Introduction
Avian Influenza Virus H9 Antigen Test is a lateral flow immunochromatographic assay for the qualitative detection of avian influenza H9 virus (AIV H9) in avian larynx or cloaca secretions.
Advantage
|
CLEAR RESULTS |
The detection board is divided into two lines, and the result is clear and easy to read. |
|
EASY |
Learn to operate 1 minute and no equipment required. |
|
QUICK CHECK |
10minutes out of results, no need to wait long. |
TEST PROCESS:
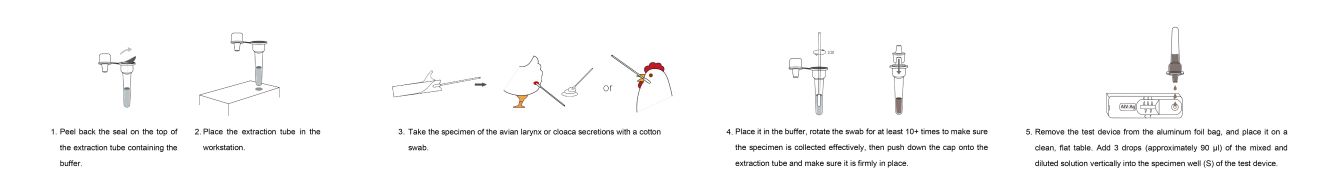
Directions For Use
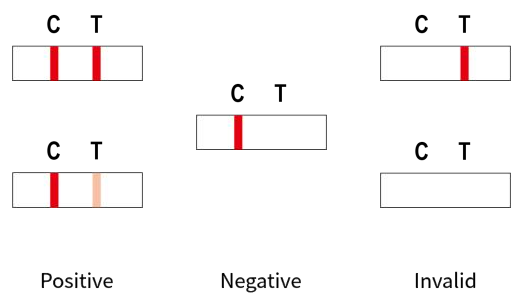
INTERPRETATION OF THE RESULTS
-Positive (+): Two colored lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region(T).
-Negative (-): Only one colored line appears in the control line region (C), and no colored line appears in the test line region (T).
-Invalid: No colored line appears in the control line region (C), indicating that the test result is ineffective. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. In this case, read the package insert carefully and test again with a new test device.