AFP Alpha-Fetoprotein Test Kit
Parameter table
| Model Number | TSIN101 |
| Name | AFP Alpha-Fetoprotein Test Kit |
| Features | High sensitivity, Simple, Easy and Accurate |
| Specimen | WB/S/P |
| Specification | 3.0mm 4.0mm |
| Accuracy | 99.6% |
| Storage | 2'C-30'C |
| Shipping | By sea/By air/TNT/Fedx/DHL |
| Instrument classification | Class II |
| Certificate | CE ISO FSC |
| Shelf life | two years |
| Type | Pathological Analysis Equipments |
Principle of FOB Rapid Test Device
For serum, collect blood into a container without anticoagulant.
Allow the blood to clot and separate the serum from the clot. Use the serum for testing.
If the specimen cannot be tested on the day of collection, store the serum specimen in a refrigerator or freezer. Bring the
specimens to room temperature before testing. Do not freeze and thaw the specimen repeatedly.

Test Procedure
1. When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test from the pouch.
2. Draw 0.2ml (about 4 drops) sample into the pipette, and dispense it into the sample well on the cassette.
3. Wait 10-20 minutes and read results. Do not read results after 30 minutes.
CONTENT OF THE KIT
1) Specimen: serum
2) Format: strip, cassette
3) Sensitivity: 25ng/ml
4) One kit includes 1 test (with desiccant) in a foil pouch
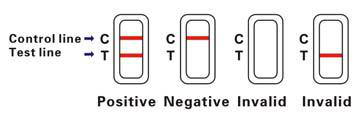
INTERPRETATION OF RESULTS
Negative (-)
Only one colored band appears on the control (C) region. No apparent band on the test (T) region.
Positive (+)
In addition to a pink colored control (C) band, a distinct pink colored band will also appear in the test (T) region.
This indicates an AFP concentration of more than 25ng/mL. If the test band is equal
to or darker than the control band, it indicates that the AFP concentration of specimen has reached
to or is greater than 400ng/mL. Please consult your physician to perform a much more detailed exam.
Invalid
A total absence of color in both regions is an indication of procedure error and/or that the test reagent has deteriorated.
STORAGE AND STABILITY
The test kits can be stored at room temperature (18 to 30°C) in the sealed pouch to the date of expiration.
The test kits should be kept away from direct sunlight, moisture and heat.
Exhibition Information






Company Profile
We, Hangzhou Testsea Biotechnology Co., Ltd is a fast-growing professional biotechnology company specialized in researching, developing, manufacturing and distributing of advanced in-vitro diagnostic(IVD) test kits and medical instruments.
Our facility is GMP, ISO9001, and ISO13458 certified and we have CE FDA approval. Now we are looking forward to cooperating with more overseas companies for mutual development.
We produce fertility test, infectious diseases tests, drugs abuse tests, cardiac marker tests, tumor marker tests, food and safety tests and animal disease tests, in addition, our brand TESTSEALABS have been well known in both domestic and overseas markets. Best quality and favorable prices enable us to take over 50% the domestic shares.
Product Process

1.Prepare

2.Cover

3.Cross membrane

4.Cut strip

5.Assembly

6.Pack the pouches

7.Seal the pouches

8.Pack the box

9.Encasement